European approval for Visanne, a drug which treats the gynecological disease endometriosis has been obtained, according to German pharmaceutical and chemical group Bayer.
European approval for Visanne, a drug which treats the gynecological disease endometriosis has been obtained, according to German pharmaceutical and chemical group Bayer.
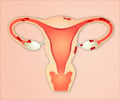
In the United States meanwhile, Bayer is still waiting for a green light for Rivaroxaban, a blood thinning treatment.Bayer said Visanne would go on sale in Europe in the second quarter of 2010, and will provide relief from pain associated with lesions and inflammation outside the uterus.
The company said the condition affects around five to 10 percent of women of childbearing age.
"Lower abdominal and pelvic pain, cramping menstrual pains (dysmenorrhea), painful sexual intercourse (dyspareunia) and infertility are well recognized symptoms of endometriosis," a statement said.
On Sunday however, Bayer said regulatory approval of Rivaroxaban in the United States had not been completed, and that the company would not be able to provide the US Food and Drug Administration (FDA) with additional information as requested, by the end of the year.
Rivaroxaban has been used in tests to treat thrombosis or blood clots following hip and knee operations.
Advertisement
Sold under the dame Xarelto in Europe, the drug is one of Bayer's best hopes for for its health-care division, along with the cancer treatement Nexavar.
Advertisement
Source-AFP
RAS