Britain’s second-biggest drug manufacturer, AstraZeneca withdrew the clinical trials of its prostate cancer drug, zibotentan, as the beneficial efficacy of the drug was proving to be questionable.
Prostate cancer, the second most common cancer affecting men, especially in the West, poses the danger of developing into non-metastatic castrate-resistant prostate tumors (CRPC) when it does not respond to treatment.
Zibotentan was expected to block the endothelin pathway that becomes uncontrolled with the advance of the disease, and thus slow down the spread of the cancer cells. Unfortunately, the ENTHUSE (Endothelin A Use) clinical trial programme did not find this happening.
There is one more study that AstraZenta hopes to finish with successful results. The study will evaluate zibotentan plus chemotherapy rather than just chemotherapy alone, in men whose cancer had spread and who have been prescribed treatment with chemotherapy.
This is not the first disappointment AstraZenta faces in the trial studies of its drugs. It had to deal with failures in Recentin in colon cancer, Vandetanib for lung cancer, Motavizumab for respiratory problems in children, and Brilinta, a blood thinning drug for heart problems.
Although AstraZeneca has led the field in its innovative medicines, observers feel that the company has to be more thorough in its research strategies.
Advertisements
Source-Medindia

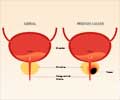
![Prostate Specific Antigen [PSA] & Prostate Cancer Diagnosis Prostate Specific Antigen [PSA] & Prostate Cancer Diagnosis](https://images.medindia.net/patientinfo/120_100/prostate-specific-antigen.jpg)