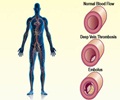
Mount Sinai School of Medicine researchers have discovered the gene variants behind stent thrombosis.
Through a partnership with the Institut de Cardiologie at Pitié-Salpêtrière University Hospital in Paris, France, the research team evaluated the DNA of 123 patients who had undergone stent implantation and developed early ST while treated with dual antiplatelet therapy, which is a combination of aspirin and clopidogrel. The patient information was shared as part of ONline ASSIstance for Stent Thrombosis (ONASSIST), a nationwide web registry of patients in France. Looking at 23 genetic variants previously associated with clopidogrel metabolism, platelet receptor function, and the control of blood clotting, they found four that were predictive risk factors of early ST. They also found that a low dose of clopidogrel in combination with a proton pump inhibitor, which is a drug to treat acid reflux, also increased the risk of early ST.
"Our research indicates that early stent thrombosis is strongly related to ineffectiveness of clopidogrel in certain patients," said Jean-Sebastien Hulot, MD PhD, Associate Professor of Medicine in the Division of Cardiology and Director Pharmacogenomics and Personalized Therapeutics at the Cardiovascular Research Center at Mount Sinai School of Medicine. "Now that we have a clearer understanding of the mechanism behind the development of stent thrombosis, we can take preventive measures to protect our patients from this deadly complication."
The research team compared the genetic code of people registered in the ONASSIST program with 246 coronary patients without early ST. They evaluated alleles, which are types of genetic variation that arise from mutations, of 23 genes. They found that CYP2C19*2 allele, which is commonly associated with loss of enzyme function, was highly prevalent in people presenting with early ST, as was the allele ABCB1 3435T, and both were infrequent in the control group. Two other alleles, CYP2C19*17 and ITGB3, were prevalent in the healthy control group but not in the early ST group indicating a protective effect. The scientists developed a genetic score where the more of these mutations that the patient had, the higher their risk for developing early ST. This risk was independent of clinical risk factors, including the use of proton pump inhibitors, acuteness of PCI, complexity of cardiac lesions, left ventricular heart function, and a high dose of clopidogrel. Only two of these risk factors- clopidogrel dose and proton pump inhibitor use - are modifiable in reducing risk for early ST. Eventually, the best prediction was achieved using the combination of both clinical and genetic factors. The authors conclude that this "clinico-genomic" approach could be useful prior to stent implantation to identify the patients with high-ST risk.
"We found that, independent of other clinical risk factors, these genetic factors play a critical role in the development of early ST," said Dr. Hulot. "Altogether, our data will help clinicians understand the factors contributing to early ST, and allow them to reduce the outcome risk to these patients. Understanding the genetic factors provides researchers with new drug targets for future study to reduce the genetic risk as well."
Mount Sinai''s Cardiovascular Research Center plans to continue the partnership with the Institut de Cardiologie at Pitié-Salpêtrière University Hospital in Paris to develop an international cardiogenomic laboratory to better understand genetic risk factors associated with heart disease and treatments for heart disease to improve and better target clinical care for patients.
Advertisement
Source-Newswise