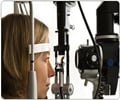
The Johns Hopkins has licensed a nationally and internationally patented formula for eye drops that could speed healing and prevent cloudiness after laser vision-correction surgery.
The Johns Hopkins University Applied Physics Laboratory (APL) has licensed a nationally and internationally patented formula for eye drops that could speed healing and prevent cloudiness after laser vision-correction surgery.
The exclusive licensing agreement allows Illinois-based AdPharma, Inc. to develop, seek regulatory approval for and market the drops, invented by APL researcher David Silver with collaborators Adrienne Csutak, András Berta and József Tõzsér of the University Medical School of Debrecen, Hungary.Studies have shown that nearly one in 10 laser-surgery patients develops hazy vision several months after the operation. In some cases the effects can last a year or longer. The APL-developed drops would prevent this “haze” from forming in the cornea. Both PRK and LASIK procedures are performed by using a laser to reshape the eye’s corneal tissue. The cornea is accessed by either removing the top layers of the cornea or by creating a replaceable flap. The drops – now in preclinical experiments – are a plasminogen activator, which stimulates a natural enzyme in tears that promotes healing in the cornea. Doctors can’t yet predict which patients will heal abnormally and suffer cloudy vision, so the researchers believe the drops could be provided to every patient after surgery, since they’re harmless to those who wouldn’t otherwise develop the haze.
Founded in 2006, AdPharma provides total drug development services, focusing on drug safety/clinical trial management and information technology services. Understanding that many promising pharmaceutical advances exist within universities and labs; the company actively seeks licenses to early stage chemical and biological research. After licensing, the company designs a comprehensive drug development plan and assumes full responsibility for its progress and success.
"Our process not only identifies the most promising work taking place in many universities and labs, but also increases the likelihood that those projects can help improve the health of people throughout the world," says Dr. Vivekananda Ramana, co-founder, chief operating officer and executive vice president of clinical affairs for AdPharma. "We are able to complement early-stage research by picking up where some organizations leave off."
Such is the case with the plasminogen activator; it's outside of APL's mission to pursue the steps beyond initial research and proof of concept, specifically clinical trials and federal Food and Drug Administration approval.
"As a research laboratory, we rely on commercial partners to complete certain development needed to transition our ideas into products," says Heather Curran, a technology and marketing manager in APL’s Office of Technology Transfer. "In this case, it was especially important that we find a partner with experience working with the FDA. This license is a wonderful example of how industry can help broaden the impact and benefit of technology developed at APL."
Advertisement
Source-Newswise
SRM/L