Massachusetts-based company BioXcell has plans to launch a new test tube approach in Britain this year, which may allow women to have a cheaper form of IVF in their lunch hour.
Massachusetts-based company BioXcell has plans to launch a new test tube approach in Britain this year, which may allow women to have a cheaper form of IVF in their lunch hour.
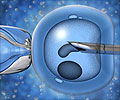
Claude Ranoux of the company has revealed that the Invocell device is designed to enable IVF to be performed without complex laboratory equipment.He says that the device may make the procedure faster, more convenient and less expensive.
He says that unlike the standard IVF wherein eggs are fertilised with sperm outside the body, the Invocell device is a sealed capsule that allows fertilisation to take place in the vaginal cavity.
As to how the procedure is performed, Ranoux says that a woman would first be given mild drugs to stimulate her ovaries, and then eggs would be removed from them while she is under sedation.
Thereafter, up to seven eggs are put into the Invocell capsule along with washed sperm, and the capsule is placed inside the vagina.
Three days later, when the patient retuns for the removal of the capsule, any fertilised embryos will be examined for quality, and the best one or two would be transferred to the womb.
Advertisement
Since eggs, sperm and embryos would at no point be stored outside the body, he says, the new procedure would help cut the cut involved in incubation.
Advertisement
During a presentation to the International Society for Mild Approaches in Assisted Reproduction conference in London, it was revealed that BioXcell has so far completed about 800 trial cycles, and recorded a clinical pregnancy rate of 19.7 per cent.
The company has applied for approval for the device from the US Food and Drug Administration, and it has also received a European Union CE mark.
Ranoux reckons that the company may launch the new technique in Britain later this year.
Many British fertility doctors, however, are doubtful as to whether the new approach would be popular.
Simon Fishel of Care Fertility in Nottingham said that a major drawback would be that embryos could not be observed immediately after fertilisation, to make sure that it has taken place normally.
He also said that the procedure might be unsuitable for about 50 per cent of IVF patients who use intracytoplasmic sperm injection (ICSI), a technique to treat male infertility.
He further said that performing such a procedure in an office setting would also be difficult due to regulatory issues.
"You would still need the accredited facilities for egg collection, and there's also the question of what you'd do with any spare embryos. If you wanted to freeze them, you'd still need an incubator and a freezer," Dr. Fishel said.
John Paul Maytum of the Human Fertilisation and Embryology Authority said: "If a clinic wanted to use this device, we would have to look very carefully at whether it would fall within our remit."
Source-ANI
SRM/L