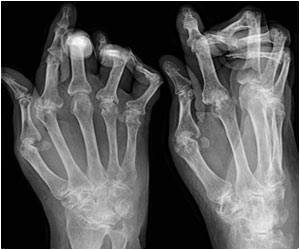
Patients with chronic gout who received the medication pegloticase showed greater improvements in uric acid measures as well as physical function and quality of life, shows study.
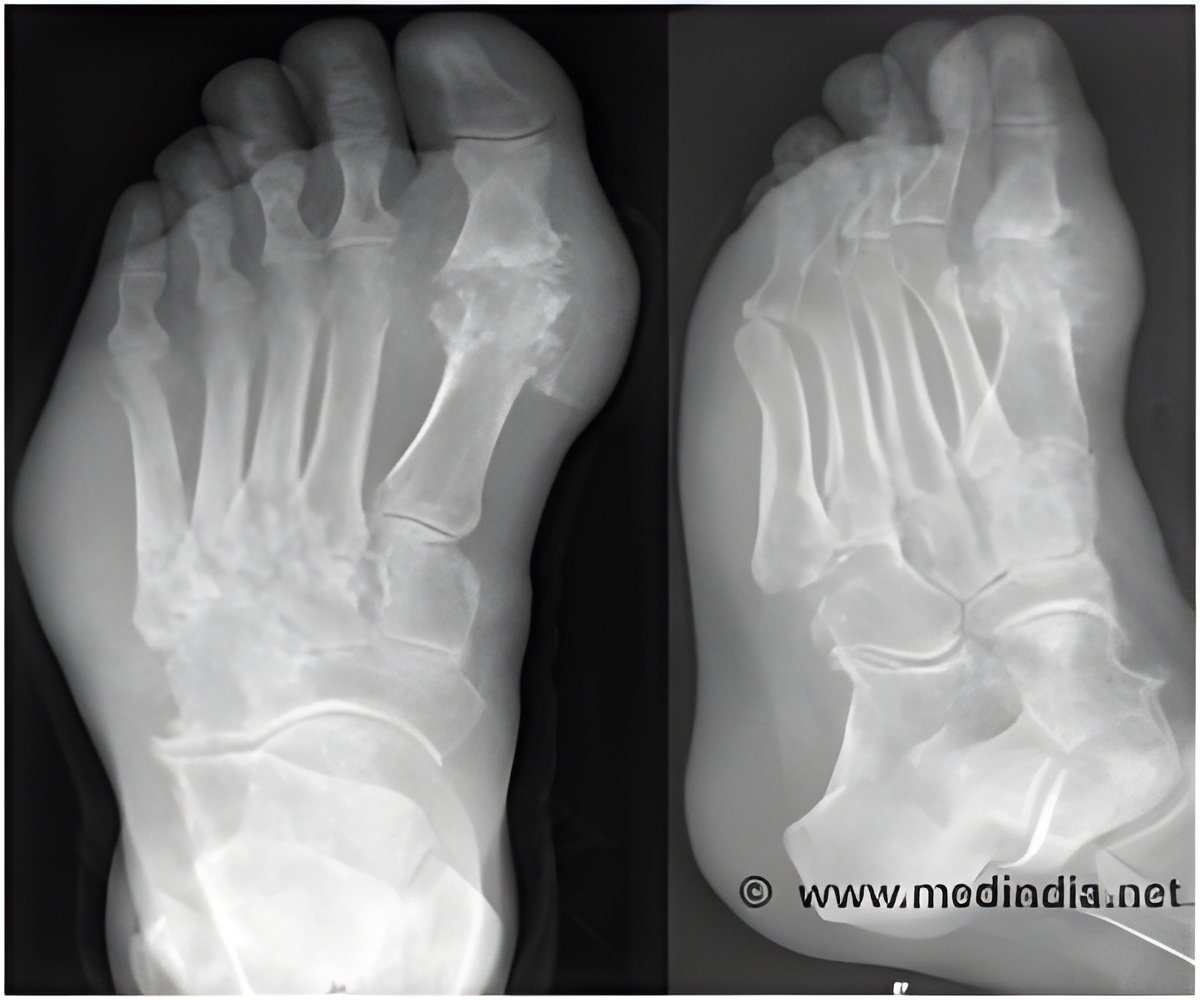
John S. Sundy, M.D., Ph.D., of Duke University Medical Center, Durham, N.C., and colleagues report results of two randomized, placebo-controlled, 6-month trials of the urate-lowering and clinical efficacy and tolerability of pegloticase in patients with refractory gout. The trials (C0405 and C0406) were conducted between June 2006 and October 2007 at 56 rheumatology practices in the United States, Canada, and Mexico in patients with severe gout, allopurinol (a drug to treat gout) intolerance or refractoriness, and serum uric acid concentration of 8.0 mg/dL or greater. A total of 225 patients participated: 109 in trial C0405 and 116 in trial C0406. Patients received 12 biweekly intravenous infusions containing either pegloticase 8 mg at each infusion (biweekly treatment group), pegloticase alternating with placebo at successive infusions (monthly treatment group), or placebo. The primary measured outcome was plasma uric acid levels of less than 6.0 mg/dL measured at months 3 and 6.
The researchers found that when analyzed separately by dose, patients treated with biweekly pegloticase experienced response rates (of meeting the primary outcome) of 47 percent (20/43) and 38 percent (16/42) in the 2 trials. Patients treated with monthly pegloticase reported response rates of 20 percent (8/41) and 49 percent (21/43), and response rates were 0 percent in both placebo groups. "When data in the 2 trials were pooled, the primary end point was achieved in 36 of 85 patients in the biweekly group (42 percent), 29 of 84 patients in the monthly group (35 percent), and 0 of 43 patients in the placebo group," the authors write. Average plasma UA for responders was substantially below 6.0 mg/dL for the entire 6-month treatment period.
The researchers also found that both pegloticase dosing groups reported significant improvements in physical function and QOL compared with placebo. Patient-reported pain was significantly reduced with biweekly pegloticase vs. placebo.
One or more adverse events (AE) occurred in more than 90 percent of participants in each treatment group. Serious AEs occurred more frequently in patients treated with biweekly (24 percent) and monthly pegloticase (23 percent) than in patients receiving placebo (12 percent). Gout flare was the most common AE and was reported in approximately 80 percent of patients across the 3 pooled study groups. Seven deaths (4 among patients assigned pegloticase and 3 in the placebo group) occurred between randomization and closure of the study database (February 15, 2008).
"These parallel, 6-month, placebo-controlled trials of pegloticase treatment have documented sustained UA reductions and significant clinical improvements in a substantial proportion of patients with chronic gout and refractoriness to, or intolerance of, conventional urate-lowering therapy. The significant disease-modifying benefits of pegloticase given every 2 weeks were demonstrable within 6 months, a time frame unique in randomized controlled trials of urate-lowering agents," the authors write.
Advertisement