New clinical guidelines for diagnosing and managing thyroid disease during this critical period have been derived from emerging data clarifying the risks of insufficient thyroid activity
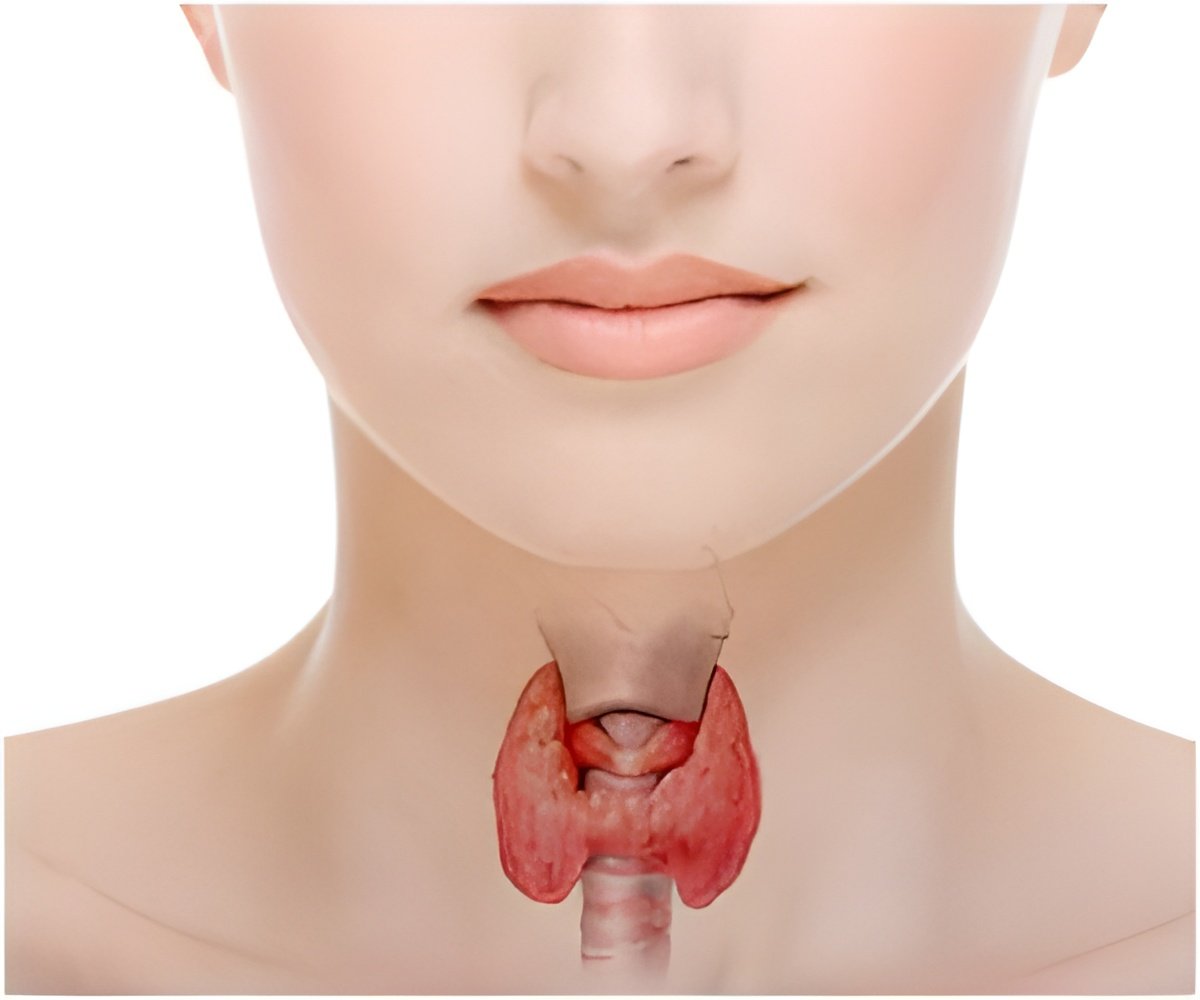
Clinical studies are producing critical data demonstrating the harmful effects not only of overt hypothyroidism and hyperthyroidism on pregnancy, but also of subclinical thyroid disease and maternal and fetal health. Ongoing research is clarifying the link between miscarriage and preterm delivery in women with normal thyroid function who are thyroid peroxidase antibody positive. Studies are also uncovering the long-term effects of postpartum thyroiditis.
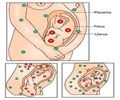
"Pregnancy has a profound impact on the thyroid gland and thyroid function…. In essence, pregnancy is a stress test for the thyroid, resulting in hypothyroidism in women with limited thyroidal reserve or iodine deficiency," state Alex Stagnaro-Green, George Washington University School of Medicine and Health Sciences (Washington, DC), and coauthors representing the ATA task force.
Among the many specific recommendations detailed in the guidelines are the following: women with overt hypothyroidism or with subclinical hypothyroidism who are TPO antibody positive should be treated with oral levothyroxine; use of other thyroid preparations such as triiodothyronine or desiccated thyroid to treat maternal hypothyroidism is strongly recommended against; and women with subclinical hypothyroidism in pregnancy who are not initially treated should be monitored for progression to overt hypothyroidism with serum TSH and free T4 measurements about every 4 weeks until 16-20 weeks gestation and at least once between 26-32 weeks gestation.
The new clinical guidelines focus on several key areas in the diagnosis and management of thyroid disease during pregnancy and postpartum: thyroid function tests, hypothyroidism, thyrotoxicosis, iodine, thyroid antibodies and miscarriage/preterm delivery, thyroid nodules and cancer, postpartum thyroiditis, recommendations on screening for thyroid disease during pregnancy, and areas for future research.
"These important guidelines were developed by a panel of international experts representing the disciplines of endocrinology, obstetrics and gynecology, and nurse midwives. This broad representation of providers that care for pregnant women will significantly increase the impact of these guidelines and translation of findings from the most recent research to clinical practice," says Gregory A. Brent, MD, Professor of Medicine and Physiology, David Geffen School of Medicine at the University of California Los Angeles and President of the ATA.
Advertisement
Advertisement