After five years of receiving infliximab (IFX) anti-TNF therapy, 61.8% of patients with ankylosing spondylitis (AS) showed substantial clinical benefit...
After five years of receiving infliximab (IFX) anti-TNF therapy, 61.8% of patients with ankylosing spondylitis (AS) showed substantial clinical benefit (ASAS40, ASsessment in AS, 40-response) and 27.6% achieved ASAS partial remission.
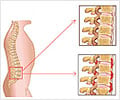
Moreover, at five years, 78.4% of AS patients had no arthritis and 84.9% had no enthesitis (inflammation at the junction between tendon and bone). Over this period, patients continued to show a sustained high response rate, low disease activity, good functional state and low creactive protein (CRP, a marker for inflammation) levels, according to the results of a new openlabel follow-up study presented today at EULAR 2009, the Annual Congress of the European League Against Rheumatism in Copenhagen, Denmark.AS is the most frequent chronic inflammatory rheumatic disease that characteristically affects the axial skeleton, entheses (sites where ligaments and tendons attach to bone) and peripheral joints. In consequence, new bone formation may result - eventually leading to ankylosis (fusion of vertebral bodies). Typical symptoms of AS include inflammatory back pain and spinal stiffness.
Dr Frank Heldmann, Centre of Rheumatology, Rheumazentrum Ruhrgebiet, Herne, Germany, the coordinator of the study, said: "Until now, our knowledge on the long-term efficacy of anti-TNF therapy in AS has remained rather limited, but the EASIC study group has significantly contributed to furthering this. Since anti-TNF agents are a relatively new treatment option for patients with AS, and can be required for long periods of time, there is strong need to know about their long-term efficacy and safety.
The data show that infliximab therapy was associated with sustained efficacy and favourable attrition rates (less than 10% per year for any reason over 5 years). Thus, our study confirms the role of infliximab as an effective and overall well tolerated treatment optoion in the management of patients with this chronic condition."
The 103 AS patients from 6 European countries (Belgium, Finland, France, Germany, the Netherlands and the United Kingdom) recruited to the 96-week open-label EASIC (European
Ankylosing Spondylitis Infliximab Cohort) trial had previously participated in the 2-year randomised controlled trial ASSERT (Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy). The EASIC population consisted of 82.5% men and 17.5% women, with a mean age of 43.5 years. There was no major difference between the EASIC and ASSERT study populations in terms of demographics or age.
Advertisement
During EASIC, all patients (except for those who had remained in remission) were continuously treated with IFX 5mg/kg (mean dosage) at intervals of 6-8 weeks (at the discretion of the investigator, according to local clinical practice). The patients in group 2 (who had also continuously received IFX between the trials) and completed the trial (n=76) showed significant improvement at week 96 compared to the ASSERT trial baseline data in a number of assessments:
- BASDAI (Bath AS Disease Activity Index): 6.4 at ASSERT baseline vs 2.5 at week 96 ofEASIC (p<0.001)
- BASFI (Bath AS Functional Index): 5.9 vs 3.1 (p<0.001)
- BASMI (Bath AS Metrology Index): 4.0 vs 2.2 (p<0.001)
- Patient global disease VAS (visual analogue score): 7.0 vs 2.8 (p<0.001)
- CRP: 2.9 mg/dl vs 0.5 mg/dl (p<0.001)
- Swollen joint count: 1.6 vs 0.6 (p<0.001)
- Enthesitis Index: 9.0 vs 0.4 (p<0.001)
Advertisement
Dr Frank Heldmann continues: "The EASIC cohort has been a success for European rheumatologists because the trial was directly organised and performed by the investigators and showed the the study drug, infliximab, to be effective and safe in long-term treatment over a period of 5 years."
Source-Eurekalert
LIN