The U.S. Food and Drug Administration has announced the launch of a study to assess the problems faced by patients after LASIK surgery.
The U.S. Food and Drug Administration has announced the launch of a study to assess the problems faced by patients after LASIK surgery.
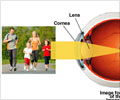
The Laser-Assisted In Situ Keratomileusis (LASIK) is a surgical procedure that uses an eximer laser to permanently change the shape of the cornea, but there have been complaints of pain and discomfort from a number of patients. And hence the study called the LASIK Quality of Life Collaboration Project, in which the FDA will be working with the National Eye Institute and the U.S. Department of Defense.Funded by the government agencies, the project is composed of three phases. The objective of Phase 1, which began in July 2009, is to design and implement a Web‑based questionnaire to assess patient-reported outcomes and evaluate quality of life issues post-LASIK, some of which may relate to the safety of the lasers used in the LASIK procedure.
Phase 2 will evaluate the quality of life and satisfaction following LASIK as reported by patients in a select, active duty population treated at the Navy Refractive Surgery Center.
Phase 3 will be a national, multi-center clinical trial and will study the impact of the procedure on quality of life following LASIK in the general population. Patient enrollment in Phases 2 and 3 have yet to begin but plans are underway. Phase 3 is expected to end in 2012.
The results of the project will help identify factors that can affect quality of life following LASIK and potentially reduce the risk of adverse effects that can impact the surgical outcome. If any of these factors are related to the safety or effectiveness of the lasers used in LASIK surgery, the FDA will evaluate whether any action is necessary. The project is part of the FDA’s ongoing effort to better monitor and improve the safety and effectiveness of the lasers used in LASIK surgery.
“This study will enhance our understanding of the risks of LASIK and could lead to a reduction in patients who experience adverse effects from the procedure,” said Dr. Jeffrey Shuren, acting director of the FDA’s Center for Devices and Radiological Health.
Advertisements
Under legislation passed in 1990, user facilities, which include nursing homes, outpatient clinics and ambulatory surgical centers, must report device-related deaths to the FDA and to the device manufacturer. They also must report device-related serious injuries to the manufacturer or to the FDA if the manufacturer is not known. Requirements include having a written protocol for adverse event reporting.
Advertisements
“Many people in the U.S. undergo LASIK procedures,” said Shuren. “Ambulatory surgical centers that perform LASIK must maintain a robust reporting system as required by law. Reporting adverse events to the FDA is critical to better understand the safety and effectiveness of ophthalmic lasers used in LASIK procedures and to enable the FDA to take appropriate actions where the lasers do not meet safety and effectiveness requirements.”
Source-Medindia
GPL