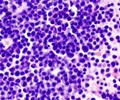
Refractory myeloma may occur in patients who never see a response from their treatment or who initially respond to treatment, but stop responding after relapse.
‘FDA approves Amgen’s drug, Krypolis in combination with Revlimid and dexamethasone for the treatment of patients with relapsed or refractory multiple myeloma.’





The drug was approved as a single agent by the FDA in 2012 and now it has expanded it into full approval.Amgen said, “The agency approved Kyprolis in combination with dexamethasone or with the drug lenalidomide plus dexamethasone for relapsed or refractory patients. It approved the drug as a single agent for patients with relapsed or refractory disease who have received one or more treatments.”
Source-Medindia