Learn about the Ten-year outcome of enzyme replacement therapy with Agalsidase beta in patients with Fabry disease.
What is Fabry’s Disease
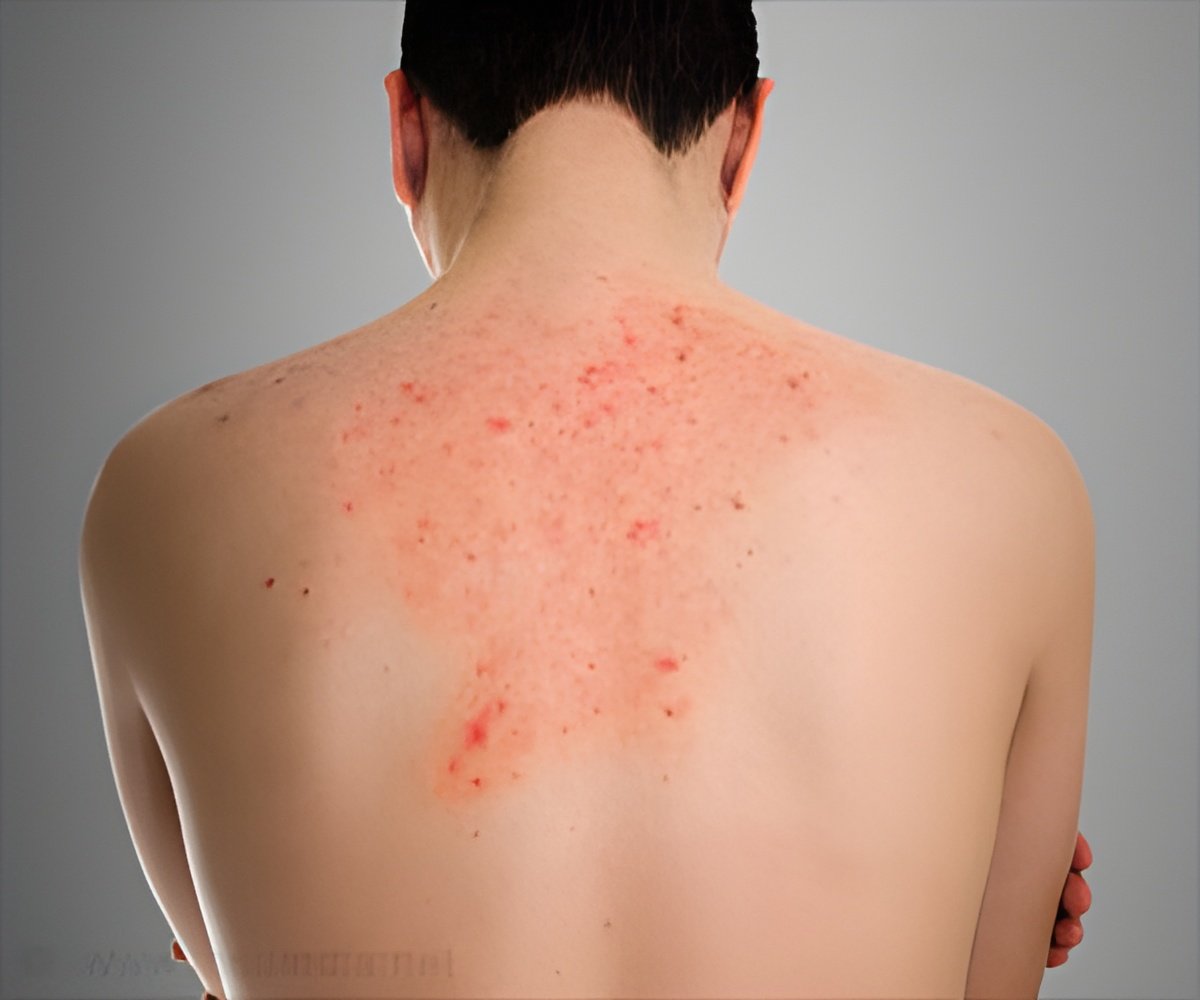
Fabry’s disease is a progressive and life-threatening genetic disease, caused by lack of specific enzyme alpha-galactosidase A (Fabryzyme). It is a rare disease and often missed by treating doctors and the incidence is estimated to be between one in 40,000 and one in 120,000 live births. Fabry disease is a "storage disorder" due to the abnormal accumulation of fat especially around the walls of blood vessels leading to decreased blood flow. This abnormal process occurs in blood vessels universally but tends to affect more the vessels in the skin, kidneys, heart, brain and nervous system.It first manifests in childhood with episodes of pain and burning sensations in the hands and feet. Painful episodes are usually triggered by exercise, fever, fatigue, stress, or even change in weather conditions. If children are examined the body shows a spotted, dark red rash on the skin (which is medically called angiokeratomas) and the distribution is usually from the umbilicus to the knees. On further questioning they would say that they do not perspire as much as their friends.
From third decade (30 to 45 years) the disease progresses to a stage when symptoms related to kidney, heart and/or nervous system start manifesting as these organs start getting compromised. Kidney failure or heart dysfunction is not uncommon and many will develop kidney failure, as the kidney’s unit called nephron gets sclerosed. In the heart there maybe mitral valve prolapse.
If kidneys are affected; the urine test may show high protein leakage.
Other symptoms may include joint and back pain, ringing in the ears, and bowel movement immediately after eating.
Cause of Fabry’s Disease
Alpha-galactosidase A is required to metabolise fatty substances including oils, waxes and fatty acids. As the body can't make enzyme alpha-galactosidase A, these fatty deposits build up in the body thereby affecting skin, kidneys, heart, brain and nervous system.Treatment for Fabry’s Disease
Enzyme replacement therapy is the only FDA approved treatment recommended for Fabry’s disease by the physicians. This enzyme supplement replaces missing or incorrect working enzyme in the body, so as to break down fatty substances in the same way as natural enzyme in the body. In addition, it helps to relieve pain and other symptoms caused by Fabry’s disease. One such enzyme is Agalsidase beta used as enzyme replacement therapy and given intravenously. However, this drug is expensive and the cost of Fabrazyme's was about US$200,000 per patient for one-year treatment.Clinical Study
Researchers carried out study to show the effectiveness of Agalsidasebeta in patients with classic Fabry’s disease.The study conducted was used to investigate long-term outcome in 52 of 58 patients with classic Fabry’s disease from phase 3 clinical trial of Agalsidase beta using aggregate data from the trial and extension study. All patients had Fabry disease confirmed by testing for alpha-galactosidase A activity and gene mutation analysis. Clinical events were defined as stroke, cardiac, renal and death (cardiac and non-cardiac). Patients were classified on the basis of renal involvement, namely as low renal involvement (LRI) and High Renal Involvement (HRI). The mean age of patients in the study was 30 years; however patients having high renal involvement were 10 years older than low renal involvement patients. Assessments were done by measuring proteinuria; left ventricular posterior wall thickness (LPWT) and interventricular septum thickness (IVST).
No significant increase was seen in left ventricular posterior wall thickness and interventricular septum thickness during the 10-year treatment period, suggesting stabilisation over time. Left ventricular posterior wall thickness and interventricular septum thickness remained stable in patients who were aged <40 years and receiving Agalsidase beta. However, in patients aged >40 years, the left ventricular posterior wall thickness and interventricular septum thickness significantly increased from baseline to follow-up.
Future Directions
Enzyme replacement therapy for such diseases has some limitations too. All patients are not suitable for treatment and problems arise in assessing efficacy for such highly variable diseases. In addition, these therapies are relatively expensive and many patients could not afford, thereby limiting access to affluent cohort.Long-term observation would be required that can further explain long term perspective of Fabry’s disease patients.