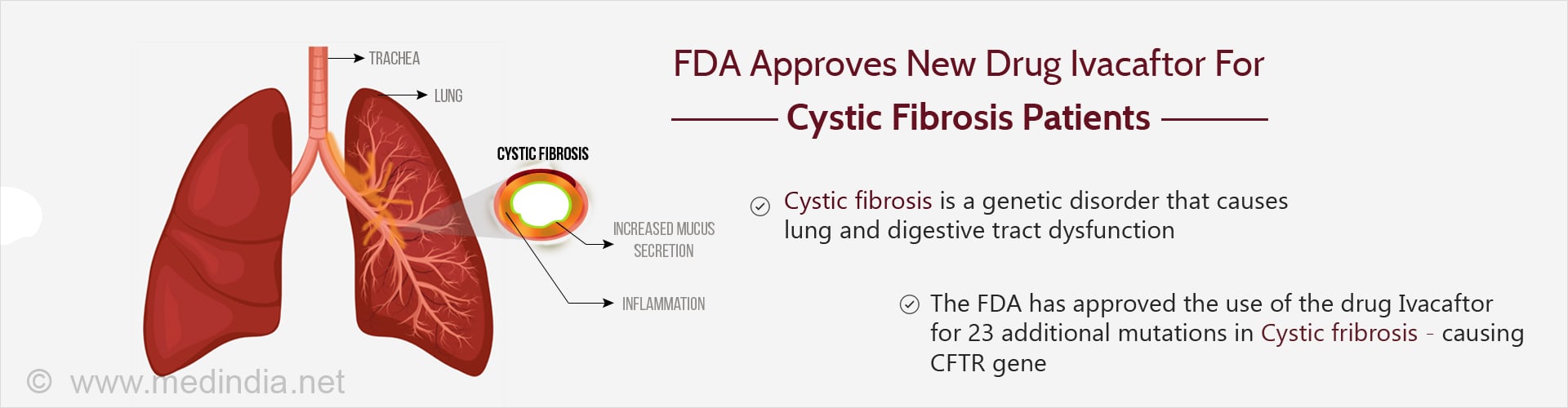
The US FDA has allowed the use of ivacaftor for 23 additional mutations in cystic fibrosis patients, thereby allowing more patients to experience its benefits.
- The new drug ivacaftor addresses the problem of chloride transport within the cells of patients with cystic fibrosis
- It is used in the treatment of patients with specific mutations in the CFTR gene
- The expansion of its use from 10 to 33 mutations will ensure that more patients will receive the benefits of this drug
Cystic fibrosis results in the accumulation of sticky mucus in several organs of the body, especially the lungs, pancreas and liver, resulting in reduced functioning of these organs. The sweat in these patients is typically salty. Treatments are aimed at reducing the symptoms, and preventing and treating complications. Newer treatments, including gene therapy, are being explored to improve the quality of life and the life span of patients affected by this genetic disorder.
The new drugs ivacaftor and the ivacaftor/lumacaftor combination address the basic problem of cystic fibrosis. They improve the function of the chloride channel that is defective in patients with cystic fibrosis and thereby improve the condition.
Ivacaftor first received approval for the treatment of cystic fibrosis due to a specific G551D mutation in the CFTR gene in 2012. It is, however, not effective in those with the F508 mutation in the CFTR gene, which is the more common mutation in cystic fibrosis. The patient can find out what type of mutation they have based on available laboratory tests for the purpose.
Ivacaftor has to be taken in tablet form or as granules twice a day with a fatty meal. Side effects include symptoms of an upper respiratory tract infection like sore throat, nasal or sinus congestion, or runny nose, headache, stomach ache, diarrhea, rash and dizziness.
With the recent FDA approval to increase the spectrum of cystic fibrosis patients who can receive ivacaftor, from 10 mutations to 33, the benefit of the drug will reach around 900 more people suffering from the genetic disorder in the United States. The additional approval is based on laboratory studies and not on clinical trials since the latter are not feasible given the relatively small number of patients suffering from the disease.
- FDA expands approved use of Kalydeco to treat additional mutations of cystic fibrosis - (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm559212.htm)
Source-Medindia