Wrinkles appear on the skin due to aging. Botox can now be used to reduce wrinkles due to crow’s feet.
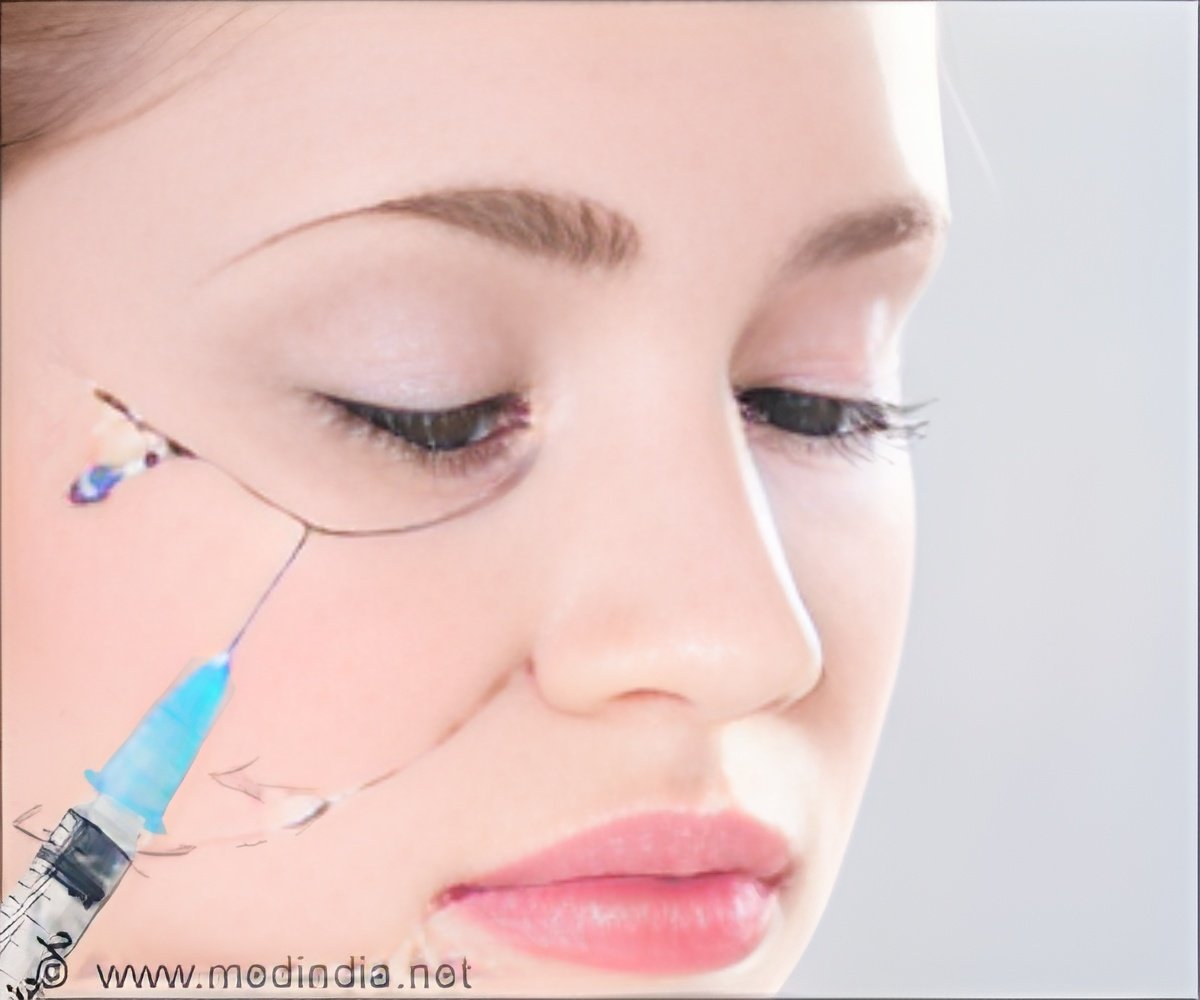
Botox has been used for quite some time for the cosmetic treatment of certain wrinkles like frown lines between the eyebrows. It is also approved for other important indications like severe spasticity of the neck, upper limb, and eyelid muscle. Injection of the toxin into the muscle relaxes the muscle. Botox has been used for severe underarm sweating. It also helps to clear out wrinkles by preventing tightening of the small muscles of the face. However, the effect is temporary and has to be repeated on a regular basis for a continuous effect.
The US FDA has recently approved Botox Cosmetic for the treatment of crow’s feet at the outer corner of the eye. Botox Cosmetic contains the form of botulinum toxin referred to as OnabotulinumtoxinA. The approval is based on recent studies that established the safety and efficacy of the product in this condition. In this case also, the effect is temporary and the injections have to be repeated on a regular basis for sustained effect.
Side Effects of Botox Injections
Since the injection is very close to the eyelids, there is a possibility that the Botox can spread to the eyelids and cause swelling. It should be administered by a trained and experienced person to reduce the chances of possible side effects of the procedure.
Some patients may be allergic to the toxin, and therefore the use of the toxin should be avoided in these patients. People with certain nerve and muscle disorders like myasthenia gravis or Lambert-Eaton syndrome could possibly suffer serious adverse effects from the injection. Therefore, the injection should be avoided in these patients.
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm367662.htm