The current guidelines for detecting melanoma are reviewed to propose risk-based, data-driven guidelines for effective screening.
Highlights
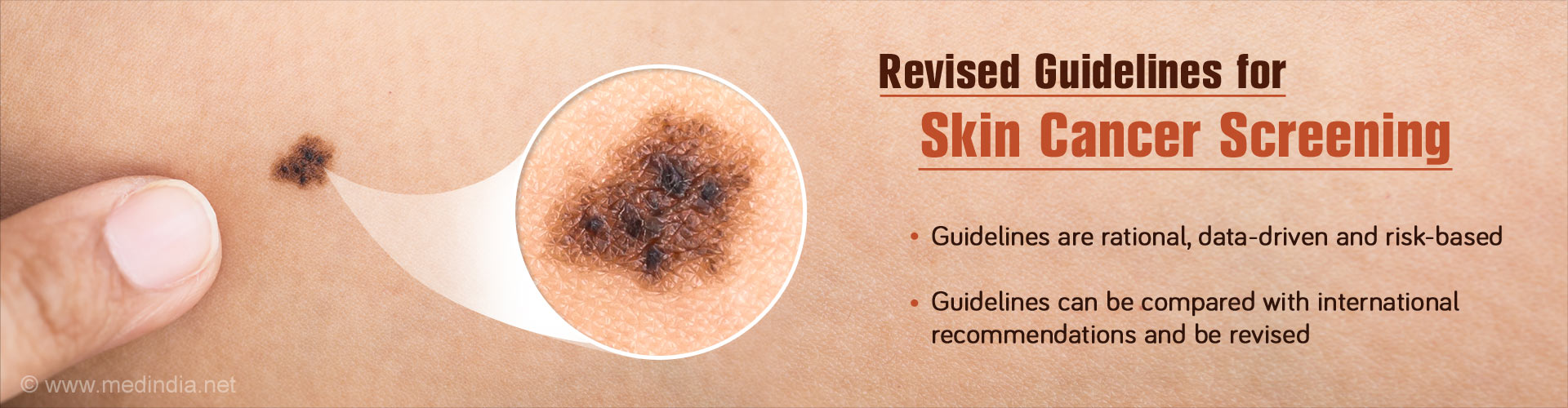
- Total body skin examination (TBSE) is the safest, easiest and possibly most cost-effective screening test for melanoma, but there is no consensus regarding its benefit or implementation.
- The recent review aims to propose rational, risk-based, data-driven guidelines for skin cancer screening.
- These guidelines are meant to serve as a starting point for further discussion and can be modified or refined .
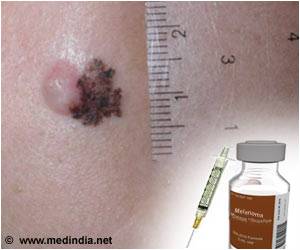
In 2016, there were an estimated 76,380 new cases of melanoma and an average of 10,130 melanoma-related deaths in the U.S. It is the fifth most common invasive cancer in men and the seventh in women.
The average 5-year survival rate is 91.5%, but it varies significantly based on the stage of disease. In the last 40 years, the incidence of melanoma has increased by nearly 200%.
The current screening practice for melanoma in primary care have been reviewed by over 50 leaders from the field of dermatology, as recommended by the US Preventive Service Task Force's (USPSTF) 2016 Draft Recommendation Statement in light of the most recent melanoma epidemiology in order to:
- propose rational, risk-based, data-driven guidelines for skin cancer screening that highlighted risk groups for melanoma and provided full screening recommendations for those individuals
- compare the proposed guidelines to recommendations made by other national and international organizations from Australia, New Zealand, Germany and other countries
A key aspect of the perspective article was the detailed analysis of the USPSTF statement and the rationale that lead to it.
"In many ways, it's surprising that our field is currently without a national consensus for skin cancer screening guidelines for patients without symptoms," stated Sancy Leachman.
"Given the current rates of melanoma diagnoses in the US, it is fascinating to see how the field continues to address screening inadequacies for a cancer with potentially such high mortality rates," commented commissioning editor for Melanoma Management, Sebastian Dennis-Beron. "It has been a pleasure to work with Dr Leachman and her colleagues to develop this timely and thought provoking piece, as to really underline the need to develop evidence-based screening guidelines for melanoma patients, given how vital early detection is for survival".
The new article is published in the Future Science Group (FSG) journal Melanoma Management.
Reference
- Sancy A Leachman et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Management; (2017) doi:10.2217/mmt-2016-0022
Source-Medindia