Using mesenchymal stem cells can be used as a novel stand-alone therapy for groups of asthma sufferers who do not respond to corticosteroid therapy.

- Induced pluripotent stem cells are a type of pluripotent stem cell that has the ability to be differentiated into a variety of tissue types.
- Mesenchymal stem cells (MSCs) //derived from induced pluripotent cells can regenerate damaged lung tissue.
- The MSCs reduced inflammation, airway remodeling and airway hyper-responsiveness in asthma induced mice.
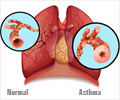
Mesenchymal Stem Cells in Asthma Treatment
The Monash Biomedicine Discovery Institute (BDI) scientists provided the experimental expertise to test Cynata Therapeutics' induced pluripotent stem cell-derived mesenchymal stem cells (MSCs) in a model of experimental asthma.
Induced pluripotent stem cells are a type of pluripotent stem cell that can be generated directly from adult cells; they have the ability to be differentiated into a variety of tissue types and, in this case, MSCs that can regenerate damaged lung tissue.
Lead researchers Associate Professor Chrishan Samuel and Dr Simon Royce tested the efficacy of the MSCs on three key components of asthma in a preclinical model of chronic allergic airways disease: inflammation; airway remodeling (structural changes that occur in lungs as a result of prolonged inflammation); and airway hyperresponsiveness (the clinical symptom of asthma).
It concluded that they may provide a novel stand-alone therapy or an adjunct therapy for groups of asthma sufferers who do not respond to current (corticosteroid) therapy.
"When we've tested other types of stem cells they haven't been able to fully reverse scarring and lung dysfunction associated with asthma - we've had to combine them with anti-scarring drugs to achieve that. These cells were remarkable on their own as they were able to effectively reverse the scarring that contributes to lung dysfunction and difficulty in breathing," he said.
Further research will be conducted to test the MSCs in combination with, or compared to a clinically-used corticosteroid. Clinical trials using the cells as a novel target for asthma are then envisaged.
Reference
- Samuel, C. S et al., Intranasal administration of mesenchymoangioblast-derived mesenchymal stem cells abrogates airway fibrosis and airway hyperresponsiveness associated with chronic allergic airways disease, FASEB Journal (2017) http://dx.doi.org/10.1096/fj.201700178R.
Source-Medindia