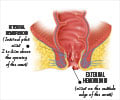
The US Food and Drug Administration (FDA) has approved a patented device called Hem-Avert® Perianal Stabilizer, designed for use during labour and vaginal childbirth to prevent hemorrhoids.
The Hem-Avert which is developed by Plexus Biomedical, is unique and designed to provide adequate support to the perianal region of a patient during labour and delivery.
This device which will be available in hospitals as well as with physicians, is a boon to nearly 750,000 to 1 million women in the Unite States who suffer external hemorrhoids after vaginal childbirth.
Source-Medindia