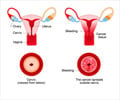
USFDA has released a new set of guidelines for the use of already approved Recombinant Human Papillomavirus 9-valent Vaccine (GARDASIL 9) in males.
‘GARDASIL 9, Merck’s Human Papilloma Vaccine can now be administered to males from 16 through 26 years of age to prevent oral cancer, precancerous lesions and genital warts.’





The vaccine prevents oral cancer caused by HPV types (16, 18, 31, 33, 45, 52 and 58), precancerous or dysplastic lesions caused by HPV types (6, 11, 16, 18, 31, 33, 45, 52 and 58) and genital warts caused by HPV types 6 and 11.GARDASIL 9 has been already approved by the USFDA for use in boys 9 through 15 years of age for the prevention of these diseases. Similarly, the vaccine can prevent cervical, vulvar, vaginal, and anal cancers caused by HPV types when administered to girls and young women 9 through 26 years of age.
GARDASIL 9 is contraindicated in individuals with hypersensitivity, including severe allergic reactions to yeast, or after a previous dose of GARDASIL 9 or GARDASIL [Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant].
Source-Medindia