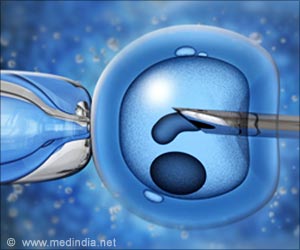
Taiwanese researchers have developed a technique that could facilitate selection of the most viable embryos to implant, leading to higher IVF success rates.
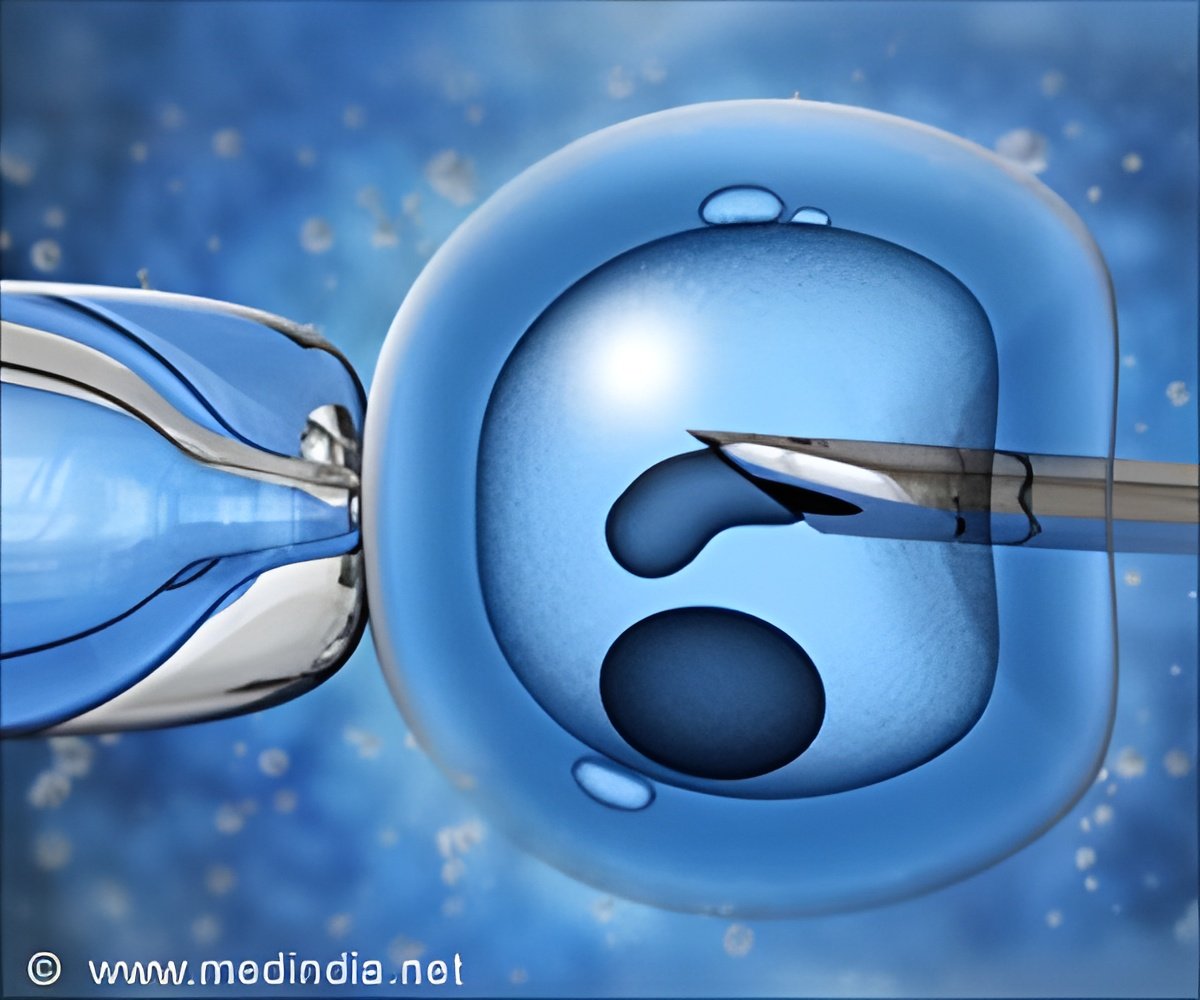
In IVF treatment, eggs are mixed with sperm in test tubes, and the fertilized eggs grow into embryos that can be implanted inside the uterus of a woman, who will carry them to term. However, the procedure can be time-consuming, costly, and emotionally draining, often requiring multiple implantation cycles before a successful pregnancy. The new technique may more effectively grow and screen embryos prior to implantation.
Often, embryos in IVF are pooled together in small drops of fluid and then transferred into the uterus. Culturing the embryos in groups is efficient, but it also makes the implantation less selective: Lab technicians cannot easily assess the viability of an individual embryo in the microdrop. The researchers instead developed a way to culture mouse embryos in a plate of open microwells, spreading them out over the plate so each well contains just one or two embryos.
A layer of oil over the top prevents embryos from moving between microwells while still allowing a micropipette to penetrate into the system to eventually transfer the embryos to the uterus. The microwell system gives each embryo its own micro-environment, allowing researchers to determine on a case-by-case basis which ones are the most viable, the study noted.
"After the experimental conditions have been optimized for human embryos and put through clinical validations, the techniques could be applied to IVF in humans," Chen said.
The findings were detailed in the journal Biomicrofluidics.
Advertisement