Zydus Cadila, the pharma giant has sought approval from the Drug Controller General of India for a new drug named Desidustat prescribed to treat anemia.
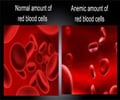
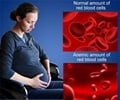
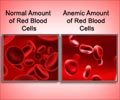
‘Desidustat is an oral alternative to injectable erythropoietin-stimulating agents (ESAs) for the treatment of anemia caused due to chronic kidney disease.’





Desidustat is an oral small molecule hypoxia-inducible factor prolyl hydroxylase (HIF-PH) for treatment of anemia in patients with chronic kidney disease. The NDA for Desidustat is based on positive data from DREAM-ND and DREAM-D Phase 3 trials in patients with chronic kidney disease.
Desidustat met its primary efficacy endpoint in both Phase 3 trials, DREAM-ND and DREAM-D, conducted in kidney patients. The data will be presented at the upcoming scientific meetings and will be published in peer-reviewed scientific journals.
"We are excited by this important milestone and thankful to all the patients, investigators, regulators and scientists, who led the discovery and development of Desidustat over the last decade. Desidustat has the potential to provide an oral, safer alternative to currently available injectable erythropoietin-stimulating agents (ESAs), by additionally reducing hepcidin, reducing inflammation, and better iron mobilisation," said Pankaj R. Patel, Chairman, Cadila.
Chronic kidney disease is a serious progressive medical condition. As per a report, 114 million people in India, 132 million in China, 38 million in the US, 21 million in Japan, and 41 million people in Western Europe are estimated to be suffering from chronic kidney disease.
Advertisement