Scientists are hoping to harness the potential of human embryonic stem cells and induced pluripotent stem cells for treating blood disorders.
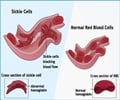
‘The reconstitution of the blood system in humans holds great therapeutic potential to treat many disorders, like blood cancers, sickle-cell anemia and others.’





In current clinical practice, this is carried out by infusing HSCs obtained from a matched donor who is immunologically compatible with the patient in need (allogeneic transplantation), or by the expansion of the patient's own HSCs in the lab, and then re-infusing them back into the patient (ex-vivo, autologous transplantation). However, the utility of both routes is currently limited by a number of factors. First, in the case of allogeneic transplantation, the scarcity of matched donors significantly increases the waiting time, which could be detrimental to the patient. Second, the ex vivo expansion of HSCs, whether allogeneic or autologous, has been a challenging task, due to the limited proliferative potential these cells exhibit in culture. These limitations have raised the need for other sources of HSCs that would alleviate the need for matched donors and yield functional HSCs in large quantities.
In 2007, Professor Shinya Yamanaka and colleagues demonstrated that somatic cells, like skin fibroblasts, could be reprogrammed back to a cellular state that resembled human embryonic stem cells (hESCs), which are a group of cells found in the blastocyst-stage human embryo and contribute solely to the development of the human fetus during pregnancy.
However, advancement in deriving functional HSCs from human pluripotent stem cells has been slow. This has been attributed to incomplete understanding of the molecular mechanisms underlying normal hematopoiesis. In this review, the authors discuss the latest efforts to generate HSCs capable of long-term engraftment and reconstitution of the blood system from human pluripotent stem cells. Stem cell research has witnessed milestone achievements in this area in the last couple of years, the significance of which are discussed and analyzed in detail.
The authors additionally discuss two highly important families of transcription factors in the context of hematopoiesis and hematopoietic differentiation, the Homeobox (HOX) and GATA proteins. These are thought of as master regulators, in the sense of having numerous transcriptional targets, which upon activation, could elicit significant changes in cell identity. The authors hypothesize that precise temporal control of the levels of certain members of these families during hematopoietic differentiation could yield functional HSCs capable of long-term engraftment.
Advertisement