For patients with thyroid eye disease, teprotumumab was found to decrease the inflammation in the eye and the build-up of tissues behind the eye that produce the long-term symptoms that reduce quality of life, stated study.
‘For patients with thyroid eye disease, teprotumumab was found to decrease the inflammation in the eye and the build-up of tissues behind the eye that produce the long-term symptoms that reduce quality of life.’





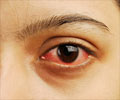
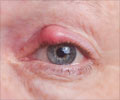
"Teprotumumab decreases inflammation in the eye and the build-up of tissues behind the eye that produce the long-term symptoms that reduce quality of life for patients with thyroid eye disease," said lead researcher George J. Kahaly, M.D., Ph.D., of Johannes Gutenberg University Medical Center in Mainz, Germany. "It offers thyroid eye disease patients new hope." The disease is associated with the outward bulging of the eye that can cause a variety of symptoms, such as eye pain, double vision, light sensitivity or difficulty closing the eye. The symptoms can lead to the progressive inability to perform important daily activities, such as driving or working. Thyroid eye disease affects more women than men.
The researchers analyzed data from two 24-week studies, with a total of 171 patients with thyroid eye disease. Prior analyses of these studies showed 77.4% of patients had a reduction in eye bulging, compared with 14.9% of those receiving a placebo, after 24 weeks of therapy. The researchers performed the new analysis to see whether patients' gender, smoking status and age influenced the drug's response rate.
The participants were randomly assigned to receive teprotumumab or a placebo. At week 24, significantly more patients receiving teprotumumab had improvements in their eye bulging compared with those who received a placebo, regardless of their gender, smoking status or age: (male: 73.1% vs. 5.0%, female: 79.3% vs. 17.9%; smokers: 70.0% vs. 23.1%, non-smokers 79.7% vs. 11.5%; younger than age 65: 76.1% vs. 16.2%, age 65 or older: 84.6% vs. 7.7%).
The average reduction in eye bulging was also significantly greater after 24 weeks of treatment in all subgroups of patients treated with teprotumumab compared with the placebo group.
Advertisement