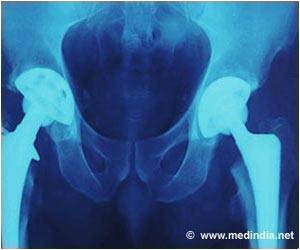
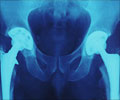
DePuy has admitted there was a manufacturing issue at its Leeds plant for metal-on-metal implants but insists that it did not have any safety implications.
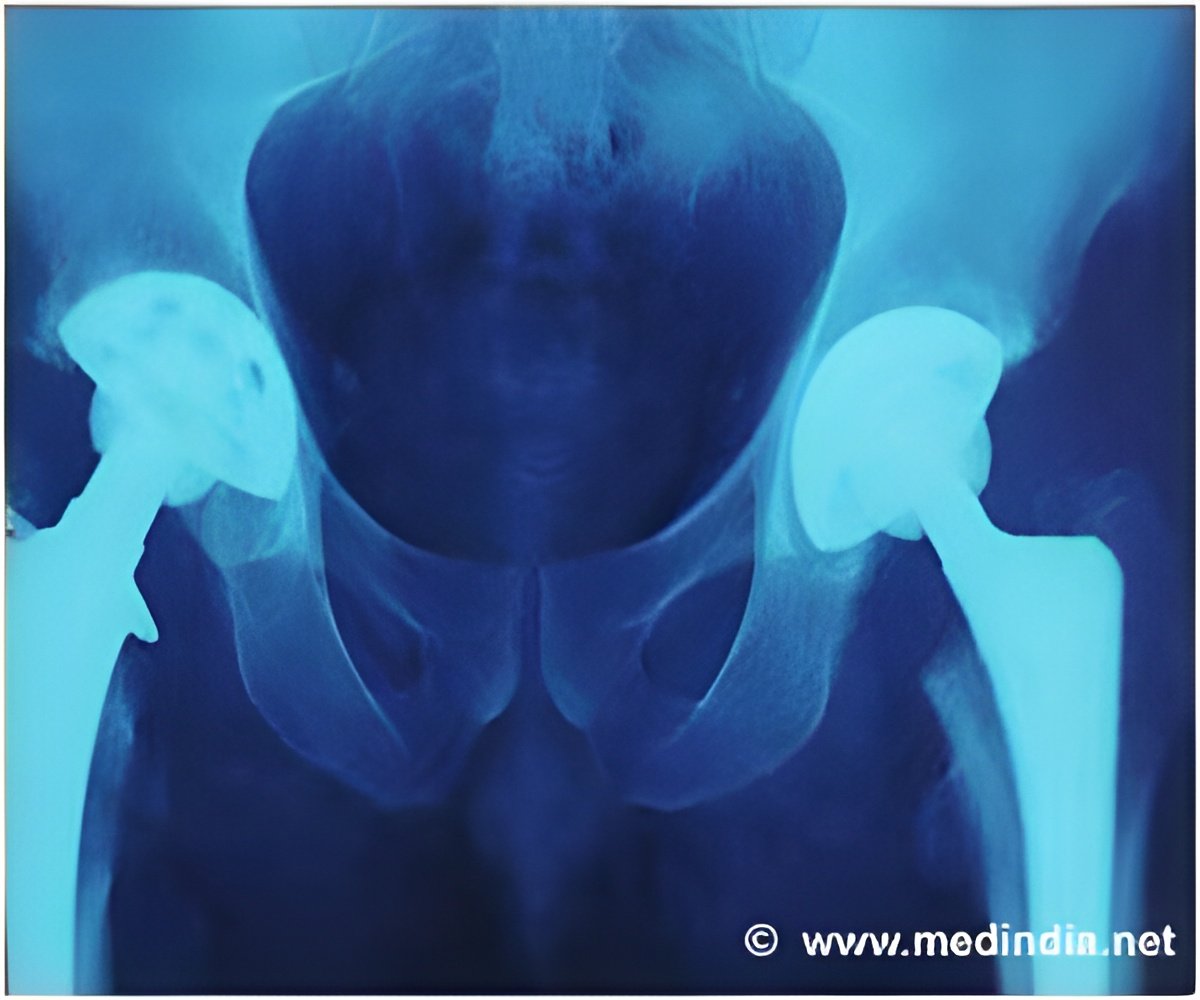
‘Hip implants manufacturer DePuy admits an error in the measuring techniques when making metal-on-metal implants, which has been used for over 23,000 patients.’





The company bosses are now under intense pressure from victims of the hip scandal to answer questions on how long the firm was aware of the problem, amid claims that potentially faulty components were sold for years.Since 2003, over 350,000 people have been fitted with at least one DePuy implant in England and Wales. Of these, over 23,000 were metal-on-metal implants. Two types of hip implants were affected by production issues, both metal-on-metal versions that are no longer used.
Health experts said that regulators should intervene and for those with the faulty implants, more regular health check-ups should be carried out and remove or replace the implants.
Metal-on-metal hip implants should be replaced sooner than other implants due to their higher wear rates – and experts have claimed that as the metal hips wear, they can deposit toxic ions into the bloodstream. It was previously believed that this problem was caused by the materials used and by hips' design. But this could be due to a potentially serious manufacturing problem.
Stephen Cannon, the honorary consultant at the Royal National Orthopedic Hospital and vice president of the Royal College of Surgeons, said that manufacturing issues could lead to problems.
Advertisement
DePuy admitted that there was a manufacturing issue at its Leeds plant but insists that it did not have any safety implications. But the US Food and Drug Administration raised concerns about the production processes for specific components as early as 2011.
Advertisement
Source-Medindia