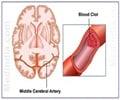
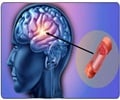
Merck’s anti-platelet drug vorapaxar has received backing from an FDA advisory committee.
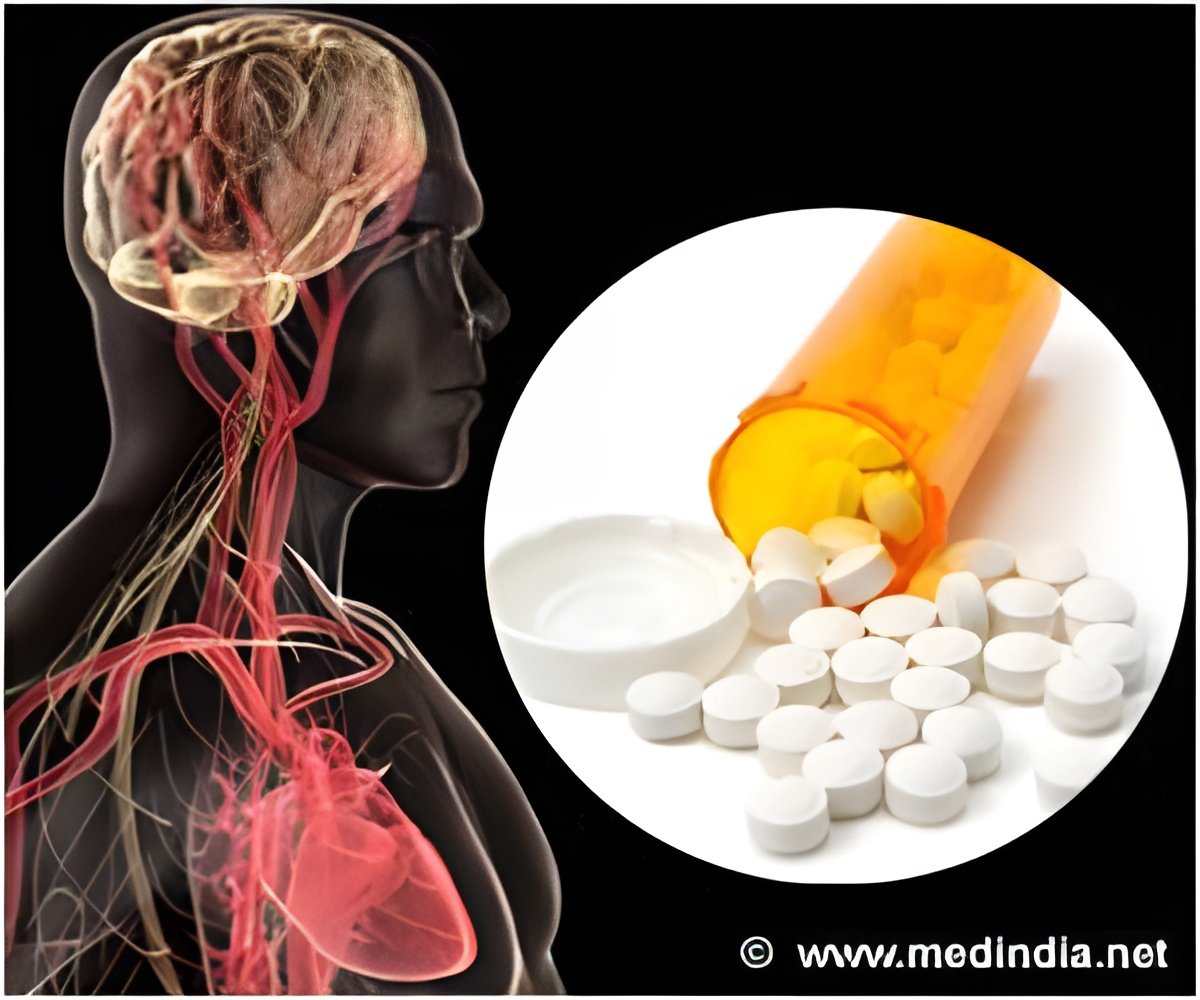
The drug has been found to cause bleeding in some patients and the lone member of the committee who voted against the approval recommendation, Mori Krantz, said that apart from bleeding, he was also not sure how many patients will actually benefit from the drug.
“I voted No, not because I didn't think the primary efficacy was met, but I worried about the size of the benefit and, in particular, with endpoints that were harder, such as cardiovascular death, where we'd need to treat nearly a thousand patients to have one person benefit”, Krantz said.
Source-Medindia