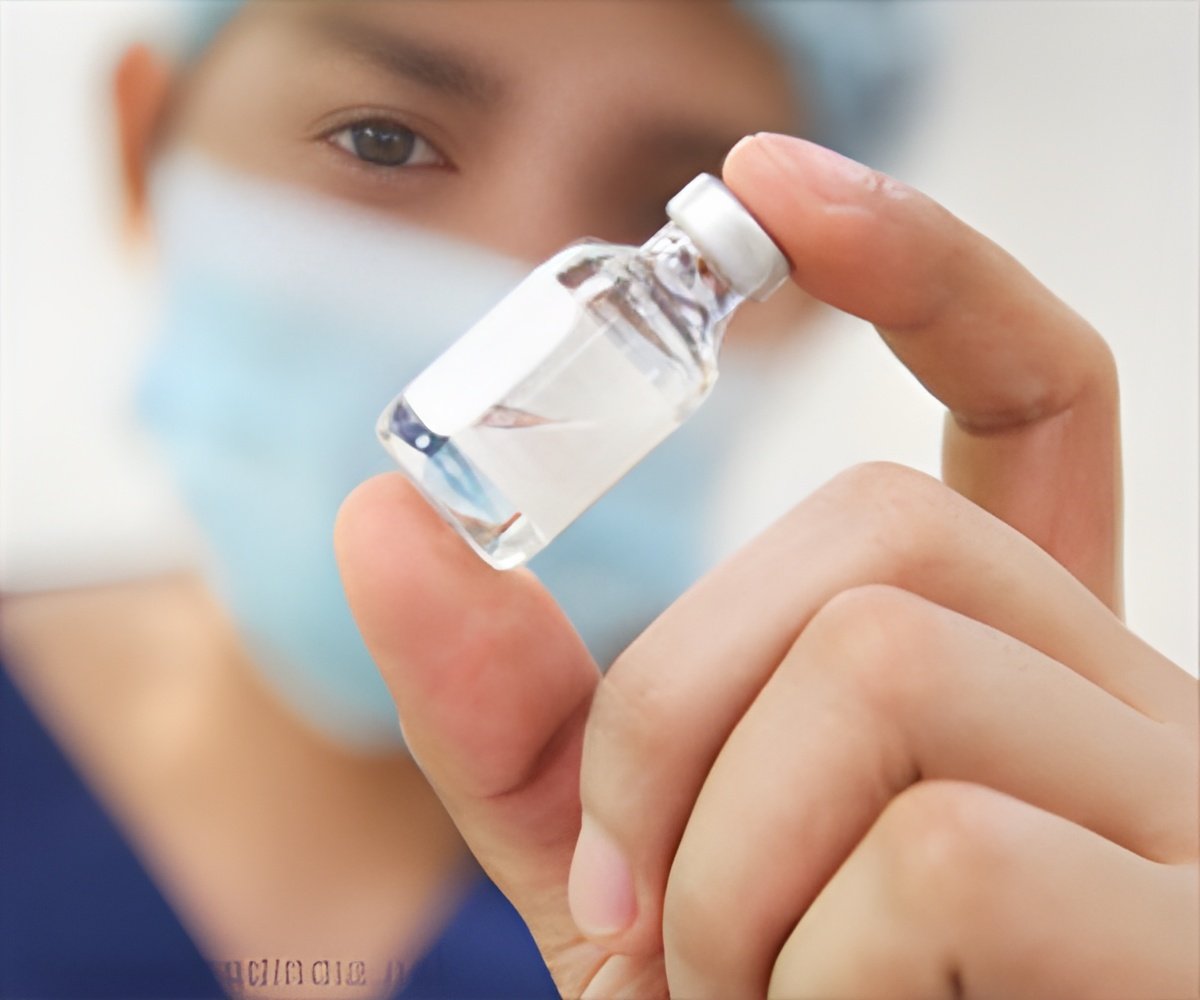
Dengavaxia, the first dengue fever vaccine produced by Sanofi has been approved for use in Mexico. Dengue fever is caused by dengue virus.
The dengue vaccine Dengvaxia, manufactured by
Sanofi, has been approved by Mexico for prevention of the deadly mosquito-borne
disease. This approval makes it the first vaccine in the world
to prevent dengue, thereby bringing fruit to the 20-year effort of the drug
company to produce the vaccine. It marks a major advance in medicine, with
dengue joining the list of vaccine-preventable diseases.
Dengue has assumed endemic proportions in several
parts of the world, claiming a huge number of lives every year. It is no longer
limited to the tropics, but has spread far and wide to other regions as well.
The virus that causes dengue spreads through the bite of the
Aedes aegypti mosquito. The fever
results in symptoms of severe joint and muscle pains. Several patients succumb
to complications like shock or
dengue hemorrhagic fever. With no definite
antiviral
treatment available against the virus as yet, a vaccine currently
seems to offer the best option to deal with the infection.
The Dengvaxia vaccine has been approved by Mexico’s
Federal Commission for the Protection against Sanitary Risk for the prevention
of dengue for people ranging from 9 to 45 years.
Since
a vaccine is administered to people who are healthy, it is important that
besides being effective, it should not cause any side effects. The vaccine has
been found to be safe in large clinical trials prior to its approval. It
provides protection against all 4 variants of the
dengue virus. Though it has not found to be 100%
effective, it will hopefully reduce the number of affected people and thereby
the transmission of the infection.
The
approval for the dengue vaccine in Mexico will hopefully be a major boost to
the global efforts involved in the prevention of dengue.
Source-Medindia