Multiple sclerosis has a potential treatment under clinical trials – An antiallergy drug and green mamba snake toxin lead the way to discovery of new drug.
‘Did You Know?
In 2020, the worldwide incidence rate of Multiple Sclerosis was recorded at 35.9 cases per 100,000 individuals. #multiplesclerosis #myelin #medindia’





In 2020, the worldwide incidence rate of Multiple Sclerosis was recorded at 35.9 cases per 100,000 individuals. #multiplesclerosis #myelin #medindia’
Advertisement
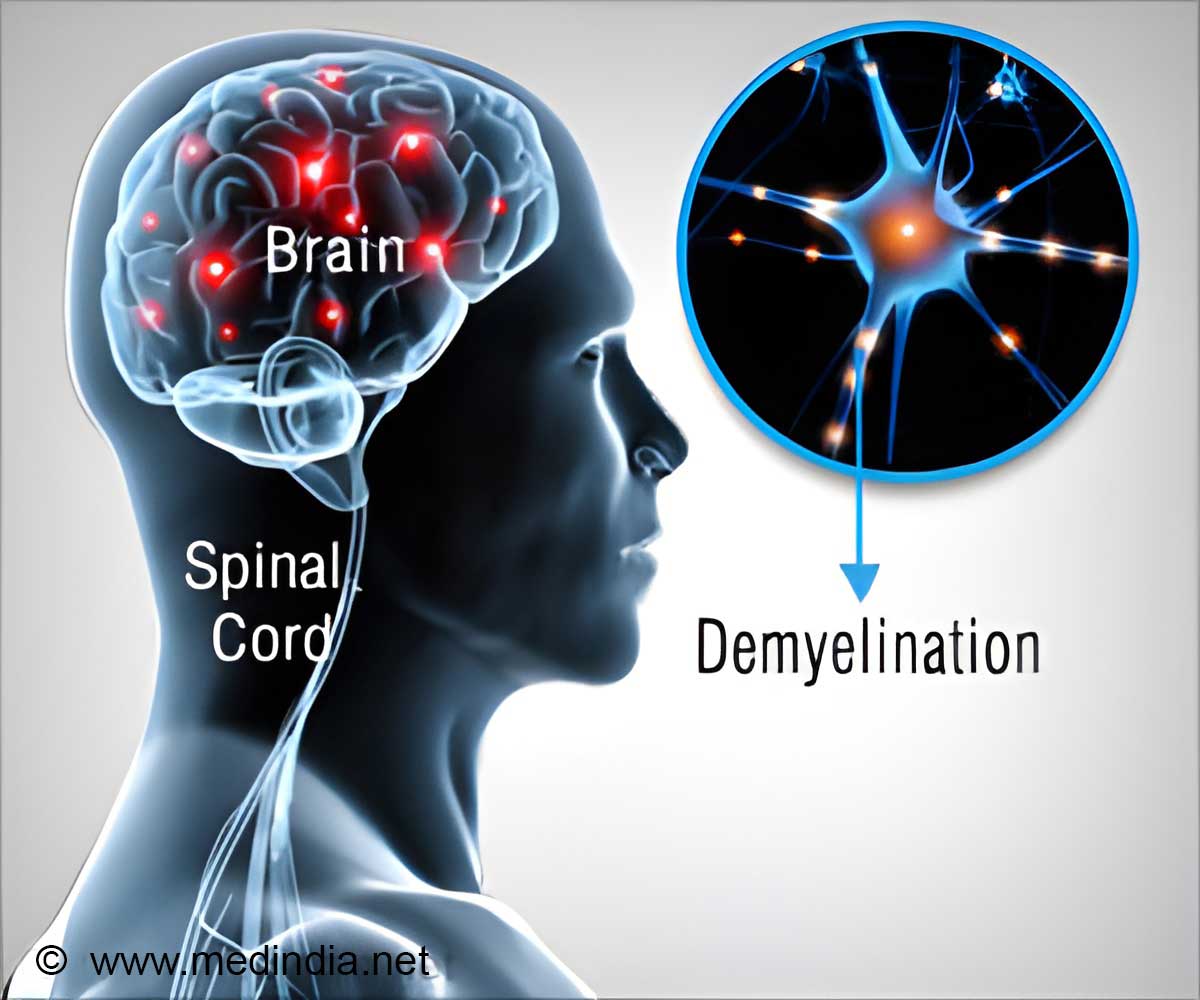
Multiple Sclerosis and its Effects
Multiple sclerosis (MS) is a neurological condition that affects both the brain and immune system. The brain is composed of a vast network of billions of neurons, which are the nerve cells responsible for transmitting signals that enable us to speak, move our limbs, and breathe. Each neuron is encased in myelin, a protective layer similar to the plastic insulation found on copper wires. However, in the case of MS, the immune system, which normally defends the body against pathogens, erroneously identifies myelin as a threat. This leads to the immune system attacking the myelin, the protective insulation around nerve cells, leaving their axons, which carry electrical impulses, exposed like bare wires. This can cause devastating problems with movement, balance and vision; and without treatment, it can lead to paralysis, loss of independence and a reduced lifespan (1✔ ✔Trusted SourceA cure for multiple sclerosis? Scientists say within our lifetime
Go to source).
Advertisement
How the New Drug Would Help Multiple Sclerosis?
Researchers at University of California, San Francisco (UCSF), in collaboration with Contineum Therapeutics, have developed a drug that encourages the body to regenerate myelin, the protective insulation that is lost. If proven effective in humans, this treatment may offer a means to reverse the damage inflicted by the disease.The new drug known as PIPE-307 targets a specific receptor found on particular brain cells, which stimulates their development into myelin-producing oligodendrocytes. When this receptor is blocked, the oligodendrocytes become activated, encasing the axons to create a new myelin sheath.
Michael Poon, PhD, a scientist at Contineum, successfully demonstrated the presence of the muscarinic acetylcholine receptor (M1R) receptor on cells capable of repairing damaged fibers. Poon achieved this using a toxin from green mamba snake venom.
The study, published on August 2 in the journal Proceedings of the National Academy of Sciences (PNAS), concludes a ten-year research effort by UCSF scientists Jonah Chan, PhD, and Ari Green, MD. In 2014, Chan led the team that discovered the potential of a little-known antihistamine, clemastine, to promote remyelination, a phenomenon previously thought to be unattainable.
“Ten years ago, we discovered one way that the body can regenerate its myelin in response to the right molecular signal, winding back the consequences of MS,” said Chan, a Debbie and Andy Rachleff Distinguished Professor of Neurology at UCSF and senior author of the paper. “By carefully studying the biology of remyelination, we’ve developed a precise therapy to activate it – the first of a new class of MS therapies.”
Advertisement
How Clemastine - An Antihistaminic, Led to the New Drug!
The original breakthrough came when Chan invented a method to screen drugs for their ability to instigate remyelination. The screen identified a group drugs, including clemastine, that had one thing in common: they blocked muscarinic receptors.Clemastine's benefits begin with its effect on oligodendrocyte precursor cells (OPCs). These cells stay dormant in the brain and spinal cord until they sense injured tissue. Then they move in and give rise to oligodendrocytes, which produce myelin.
For some reason during MS, OPCs gather around decaying myelin but fail to rebuild it. Chan figured out that clemastine activated OPCs by blocking muscarinic receptors, enabling the OPCs to mature into myelin-producing oligodendrocytes.
Nerves and their myelin are notoriously hard to repair, whether due to MS, dementia or other injury. Green and Chan carried out a trial of clemastine in patients with MS, and it was a success – the first time that a drug showed the capacity to restore the myelin lost in MS. Despite being safe to use, however, clemastine was only modestly effective.
“Clemastine is not a targeted drug, affecting several different pathways in the body,” said Green, Chief of the Division of Neuroimmunology and Glial Biology in the UCSF Department of Neurology and co-author of the paper. “But from the get-go, we saw that its pharmacology with muscarinic receptors could point us toward the next generation of restorative therapies in MS.”
A Snake Toxin Guided to the Target Receptors!
The researchers continued using clemastine to understand the curative potential of regenerating myelin in MS. They developed a series of tools to monitor remyelination, both in animal models of MS and in MS patients, showing that the benefits seen with clemastine came from remyelination – and pointing the way for how new drugs should be tested and evaluated.They also found that clemastine’s benefits came from blocking just one of the five muscarinic receptors, M1R, but the effect on M1R was middling, and the drug also affected the other receptors. The ideal drug would need to zero in on M1R.
At this point, the UCSF scientists needed an industry partner to advance the project. Ultimately, Contineum Therapeutics (then known as Pipeline Therapeutics) was formed to take a meticulous approach to creating that ideal drug. Chan and Green helped the company confirm that M1R was the right target for a remyelinating drug, and then make a drug that blocked it exclusively. Poon, a biologist at Contineum, realized that MT7, a toxin found in the venom of the deadly green mamba snake, could reveal exactly where M1R was in the brain.
“We needed to prove, beyond doubt, that M1R was present in OPCs that were near the damage caused by MS,” Poon said. “MT7, which is exquisitely selective for M1R, fit the bill.”
Poon used MT7 to engineer a molecular label for M1R that revealed rings of OPCs gathering around damage in a mouse model of MS and in human MS tissue.
New Drug and its Clinical Trials – A Potential for Halting and Curing the Disease
A team of medicinal chemists at Contineum, led by Austin Chen, Ph.D., then got to work on the drug that Chan and Green envisioned, designing PIPE-307 to potently block M1R and get into the brain.The researchers tested the effects of the new drug on OPCs grown in petri dishes and the animal models of MS using Chan’s and Green’s methods for tracking remyelination. PIPE-307 blocked the M1R receptor much better than clemastine; prompted OPCs to mature into oligodendrocytes and begin myelinating nearby axons; and it crossed the blood-brain barrier. But most tellingly, it reversed the degradation seen in a mouse model of MS.
“A drug might seem to work in these abstract scenarios, affecting the right receptor or cell, but the key finding was actual recovery of nervous system function,” Chan said.
In 2021, PIPE-307 passed a Phase I clinical trial, demonstrating its safety. It is currently being tested in MS patients in Phase II.
If it succeeds, it could transform how MS is treated.
“Every patient we diagnose with MS comes in with some degree of pre-existing injury,” Green said. “Now we might have a chance to not just stop their disease, but to also heal.”
Reference:
- A cure for multiple sclerosis? Scientists say within our lifetime - (https:www.universityofcalifornia.edu/news/cure-multiple-sclerosis-scientists-say-within-our-lifetime)
Source-Euralaert