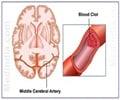
The anticoagulant, Pradaxa which is administered to reduce the risk of stroke in people who suffer atrial fibrillation has been approved by the Food and Drug Administration in the US.

"People with atrial fibrillation are at a higher risk of developing blood clots, which can cause a disabling stroke if the clots travel to the brain," Dr Norman Stockbridge, director of the FDA's division of cardiovascular and renal products, said in a statement.
Pradaxa, marketed by Germany's Boehringer Ingelhei, is an anticoagulant that works to neutralize thrombin, an enzyme essential in blood coagulation.
The drug's safety and efficacy was tested in clinical studies comparing it with warfarin.
Praxada does not require patients to have regular blood testing.
But like other anti-clotting drugs, it can have adverse effects, including life-threatening bleeding, gastrointestinal symptoms, stomach pain, nausea, heartburn and bloating.
Advertisement