- Metabolic Alkalosis - (http://jasn.asnjournals.org/content/11/2/369.full)
What is Alkalosis?
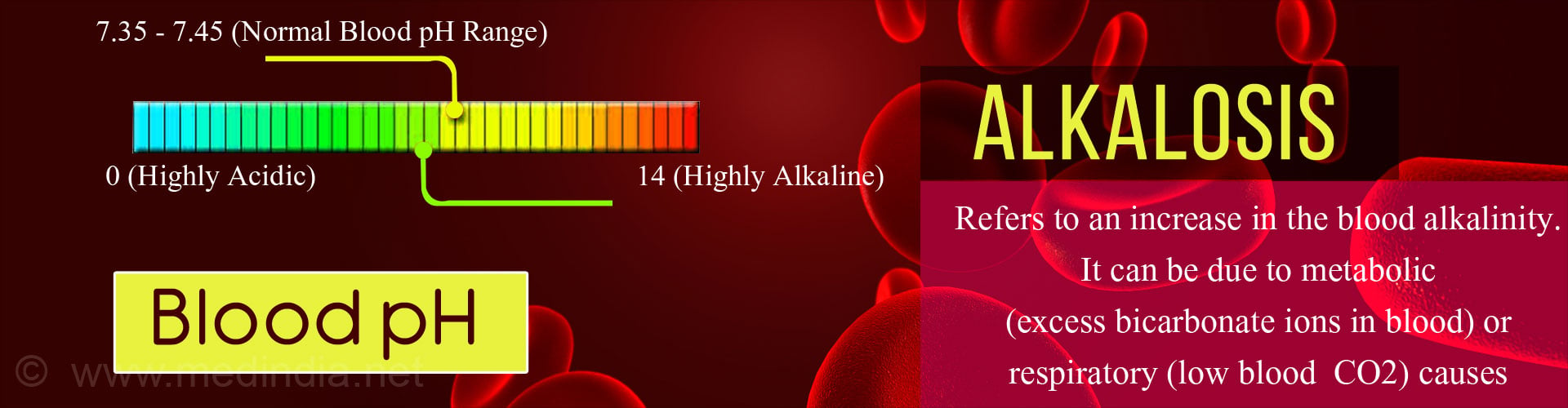
An important property of the blood is its pH which is an indication of it’s acidity or basicity (alkalinity). The pH of blood ranges from 0 (highly acidic) to 14 (highly alkaline). A pH of 7 is neutral. Blood is normally slightly alkaline, with a normal pH range between 7.35 to 7.45. Usually the body maintains the pH of blood close to 7.40.
Alkalosis is said to occur when the alkalinity of the blood increases. This may occur due to a fall in the acid level (or hydrogen ions) or a rise in the base level (bicarbonate ions).
How Does the Body Regulate the Acid Base Balance?
Physiological mechanisms within the body try to maintain the pH of blood close to 7.4. These include namely
- The lungs
- The kidneys
- Buffer systems
Role of the Lungs
One of the mechanisms of the body to control blood pH involves regulating the CO2 (carbondioxide) content of the blood.
Carbon dioxide, which is mildly acidic, is a waste product of normal cell metabolism and is thus constantly being produced by cells. The blood transports carbon dioxide to the lungs, where it is breathed out.
If carbon dioxide accumulates in the blood for unknown reasons, the pH of the blood falls (acidity increases) and if there is too little CO2, the blood becomes alkaline..Special control systems in the brain control the speed and depth of breathing (ventilation), thereby regulating the amount of carbon dioxide that is exhaled.
The brain and lungs thus regulate the blood pH every single minute by adjusting the rate and depth of breathing. For example, if the breathing is faster and deeper, more CO2 is eliminated and blood pH increases.
Role of the Kidneys
The kidneys influence blood pH by eliminating excess acids or bases in the urine. However, because the kidneys produce these changes more slowly than the lungs do, this mechanism generally takes several days.
Buffer Systems
Another mechanism for regulating blood pH involves the use of buffer systems, which protect against sudden changes in blood acidity and alkalinity. The pH buffer systems are combinations of weak acids and weak bases which are normally present in the body.
The most important pH buffer system in the blood includes carbonic acid (a weak acid formed when carbon dioxide dissolves in blood) and bicarbonate ions (the corresponding weak base).
What are the Types of Acid Base Disorders?
Acid base disorders are of 2 broad types namely
- Acidosis – Blood pH falls or becomes acidic
- Alkalosis – Blood pH increases or becomes alkaline
Metabolic acidosis or metabolic alkalosis results when the kidneys fail to produce or excrete acids or bases necessary to maintain blood pH.
Respiratory acidosis or respiratory alkalosis results when the lungs are unable to regulate pH efficiently by effecting changes in carbon dioxide exhalation.
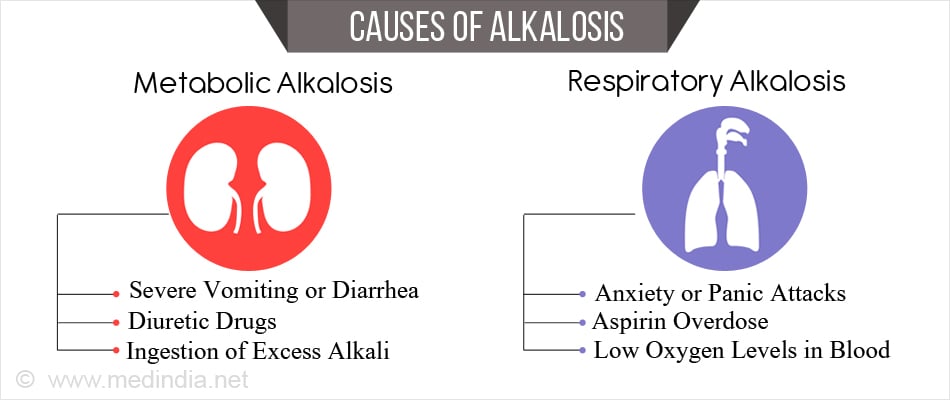
What are the Causes of Alkalosis?
Metabolic Alkalosis
Metabolic alkalosis occurs when the body loses large amounts of acid or gains a lot of bases. Some important conditions include
- Loss of acid from the digestive tract due to severe vomiting or diarrhea
- Diuretic drugs (cause loss of potassium in the urine, affecting the kidney’s capacity to maintain normal acid base balance)
- Ingestion of excess alkali
- Adrenal tumors (cause potassium loss in the urine)
Respiratory alkalosis
Respiratory alkalosis occurs when the lungs expel too much carbon dioxide which occurs due to hyperventilation. The main causes include
- Anxiety or panic attacks
- Aspirin overdose (in the initial stages)
- Low oxygen levels in blood
- Severe pain
- Fevers and infections
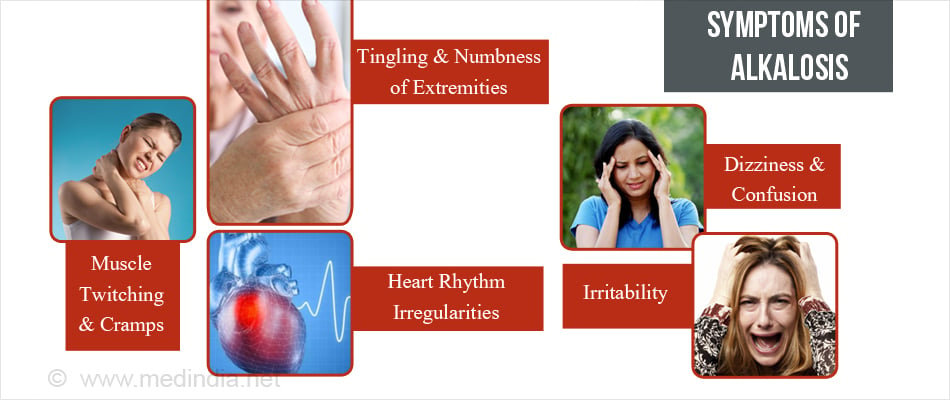
What are the Signs and Symptoms of Alkalosis?
The clinical features of alkalosis are not specific and depend on the severity of the underlying electrolyte disturbance. Occasionally there may be a paucity of symptoms. Clinical features include:
- Muscle twitching and cramps (caused by low calcium)
- Tingling and numbness of extremities (seen in hyperventilation caused by anxiety)
- Heart rhythm irregularities ( if associated with low potassium levels)
- Dizziness and confusion
- Irritability
- Seizures
- Coma in severe cases
How do you Diagnose Alkalosis?
Medical History
History may be helpful in arriving at a diagnosis. Things to ask for in history when suspecting alkalosis include:
- History of vomiting and diarrhea
- Drug history – diuretics, corticosteroids
- Age of onset of symptoms and family history (inherited disorders such as Barter’s syndrome that begin in childhood)
- History of intestinal surgery eg. ileostomy
- History of renal disease
Physical examination may reveal some of the features mentioned above.
Laboratory tests are performed to confirm the diagnosis of alkalosis and identify the cause.
Blood tests
- Arterial blood gas (ABG) analysis which includes arterial oxygen and carbon dioxide measurements and pH measurements
- Measurement of serum electrolytes including calcium, potassium and magnesium
- Serum bicarbonate levels
From the above tests the type of acid base disorder present can be diagnosed.
Urine tests
Usually the cause is evident from the history and physical examination; but if they are not contributory, and in the presence of a normal renal function, urinary Cl− and K+ concentrations are measured (values are not diagnostic in renal insufficiency).
Urine Chloride levels
Low urinary Cl (< 20 mEq/L) indicates significant reabsorption of Cl- by the kidneys and hence a Cl-responsive metabolic alkalosis. Urinary Cl > 20 mEq/L indicates chloride unresponsive alkalosis.
Urine Potassium levels
- In a patient with normal blood pressure, a reduced urinary potassium indicates hypokalemia or increased loss in stools (laxative abuse).
- Increased urinary potassium in the absence of hypertension suggests diuretic overdose.
- If urine potassium is high in the presence of hypertension, the patient must be investigated further for conditions that cause excess aldosterone to be secreted. Tests to be done include plasma renin activity, and measuring aldosterone and cortisol levels.
How do you Treat Alkalosis?
Metabolic alkalosis
- The underlying cause should be identified and treated.
- Fluids should be replaced by intravenous infusions.
- Electrolyte disturbances such as low serum potassium (hypokalemia) should also be corrected.
- Even in case of severe metabolic alkalosis, dilute acid is not commonly administered intravenously.
Respiratory alkalosis
- Generally respiratory alkalosis is not serious or life threatening. The first step is to make sure that the patient is getting enough oxygen.
- If the hyperventilation was caused due to pain, giving pain killers will usually be enough.
- If respiratory alkalosis was caused by anxiety, reassurance and calming the patient is necessary. The patient is asked to take slow and deep breaths consciously.
- Breathing into a paper bag may help as the patient breathes back the exhaled CO2 from the bag.
- As the condition is generally not serious, no interventions to lower the pH are required.
What are the Complications of Metabolic Alkalosis?
If metabolic alkalosis is not treated in the early stages and becomes severe, any or all of the following complications may occur
- Heart failure
- Seizures
- Coma
How do you Prevent Alkalosis?
Some of the measures to prevent alkalosis include:
- Avoid overuse of laxatives
- Avoid diuretic overdose
- Patients receiving tube or parenteral nutrition should have their electrolytes monitored
- Prompt treatment of severe vomiting and diarrhea with fluids and electrolytes
- Learning to be calm under stressful situations.