Sarilumab Medication Information
Discover comprehensive details about Sarilumab, including its pronunciation, uses, dosage instructions, indications, and guidelines on how and when to take it or avoid it.
The updated prescription information covers potential side effects, precautions, warnings, and storage recommendations.
Additionally, explore the Sarilumab brands available in India and internationally, along with pricing information. For personalized advice, consult your healthcare provider.
Generic Name : Sarilumab Pronunciation : sar-IL-ue-mab Therapeutic Classification : AntirheumaticsBrand Names or Trade Names of Sarilumab
India :
Kevzara
Overview of Sarilumab
• Sarilumab is an interleukin-6 receptor monoclonal antibody approved by FDA in May 2017 for the treatment of rheumatoid arthritis (RA) in adults.Why is Sarilumab Prescribed? (Indications)
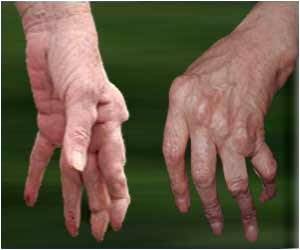
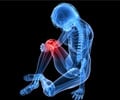
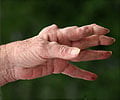
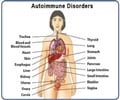
Sarilumab is prescribed for the treatment of moderate-to-severe rheumatoid arthritis in adult patients who have not shown adequate response either to one or more disease-modifying antirheumatic drugs (DMARDs).Rheumatoid arthritis is an autoimmune disease in which the body’s immune system mistakenly attacks its own joints resulting in pain, swelling, and inflammation.
Sarilumab is used alone or in combination with methotrexate or other DMARDs.
It works by blocking the activity of interleukin-6, a cytokine substance that is responsible for causing inflammation.
When should Sarilumab not be taken? (Contraindications)
Sarilumab injection should not be recommended in patients with:• Allergy to the drug
• Hepatitis or active liver disease
• Absolute neutrophil count less than 500 per mm3, platelet count less than 50,000 per mm3 or liver enzyme AST or ALT (SGOT or SGPT) above 1.5 times the upper limit of normal range.
• Weak immune system or cancer
• Presence of active infections such as tuberculosis, candidiasis, Pneumocystis, or histoplasmosis.
The risks and benefits of the drug should be evaluated before using in patients previously exposed to tuberculosis or lived or traveled to endemic tuberculosis areas.
What is the dosage of Sarilumab?
Treatment of moderate to severe rheumatoid arthritis:• The recommended adult dose of sarilumab is 200mg once for every two weeks given as a subcutaneous injection.
• If reduced neutrophil or platelet count or an increase in the liver enzymes is noted, the treatment may have to be stopped till some recovery occurs, and then resumed at a lower dose and increased to the normal dose if clinically appropriate.
How should Sarilumab be taken?
• Sarilumab comes as a clear solution in a prefilled syringe of 200mg/1.14ml or 150mg/1.14ml which should be given by a subcutaneous route just under the skin.• The injection should be removed from the cold storage 30 minutes before administration and allowed to reach room temperature. However, warming techniques such as placing it in warm water, exposing to sunlight, or heating in an oven should not be employed.
• The injection should be checked for its seal, clarity, or any visible matter before injecting. Sarilumab should not be injected into the tender, damaged, scarred or bruised skin.
• The injection sites are thigh, upper arm, and abdomen either 2 inches or 5 cm away from the navel area. Rotation of injection sites is advised.
What are the warnings and precautions for Sarilumab?
• Sarilumab may increase the risk of infection by lowering the immunity. Therefore, patients with any symptoms of infection should report to the doctor instantly for further evaluation and treatment.• Patients are required to test for latent tuberculosis (where patient is infected with Mycobacterium tuberculosis but does not have active tuberculosis). If found positive, tuberculosis treatment should be initiated before sarilumab. Patients should be monitored closely for tuberculosis infection during treatment even if they were negative for latent tuberculosis.
• If the patient shows any sign or develops a serious infection, the treatment with sarilumab must be stopped until the infection comes under control.
• Incidence of infection rate is higher in the elderly population and caution is required in treating these groups.
What are the side effects of Sarilumab?
Gastrointestinal: Severe stomach pain, black or bloody stools, vomitingCardiovascular system: Chest pain or tightness in the chest
Central Nervous System: Dizziness or fainting
Respiratory: Sorethroat, stuffy or runny nose, wheezing or difficulty in breathing, coughing up blood, pneumonia
Skin: Rash, hives, blistered or peeling skin, red or swollen skin (cellulitis), injection site reactions such as erythema and pruritus
Others: Serious infections, opportunistic infections like candidiasis and pneumocystis, urinary tract infection with blood in urine, difficulty or burning sensation while passing urine, muscle pain, fever, weakness, swelling of lips, tongue or face due to allergic reaction, increase in liver enzymes.
What are the other precautions for Sarilumab?
• Monitoring of blood counts (mainly for a neutrophil count and platelet count), liver enzymes, blood cholesterol levels are required during 4 to 8 weeks after starting the treatment and for every 3 months during the therapy.• Patients are advised to report at regular intervals to their rheumatologist for monitoring the improvement in the disease and sign of infection, if any.
What are the Drug Interactions of Sarilumab?
• Concurrent use of live vaccines such as MMR, polio, typhoid or varicella vaccine with sarilumab must be avoided due to a risk of infections.• Efficacy of simvastatin is reduced when sarilumab is taken together.
• Gastrointestinal perforations (tear of the stomach or intestinal wall) may occur in patients taking sarilumab along with NSAIDs (non-steroidal anti-inflammatory drugs) or steroids. Therefore, patients are advised to report to the physician immediately in case of stomach pain.
• Intake with warfarin could affect bleeding and clotting times.
What are the storage conditions for Sarilumab?
• Sarilumab should be stored in a refrigerator at 2°C to 8°C in the original carton. The injection should not be frozen or shaken and should be protected from light.• The injection can be stored at a controlled room temperature, not above 25°C but should be used within 14 days and to be discarded thereafter.