The strategic funding positions Amplexd Therapeutics to reshape the landscape in HPV and cervical precancer treatments with two topical, non-invasive options as potential alternatives to the “watch and wait” approach for low-grade CIN, and invasive surgery for high-grade CIN. Amplexd is poised to move the needle with the development of two low-cost therapies that favor patient comfort and increase access, and a vision to provide accessible solutions to target the often-overlooked public health crisis of HPV-induced cervical precancers.
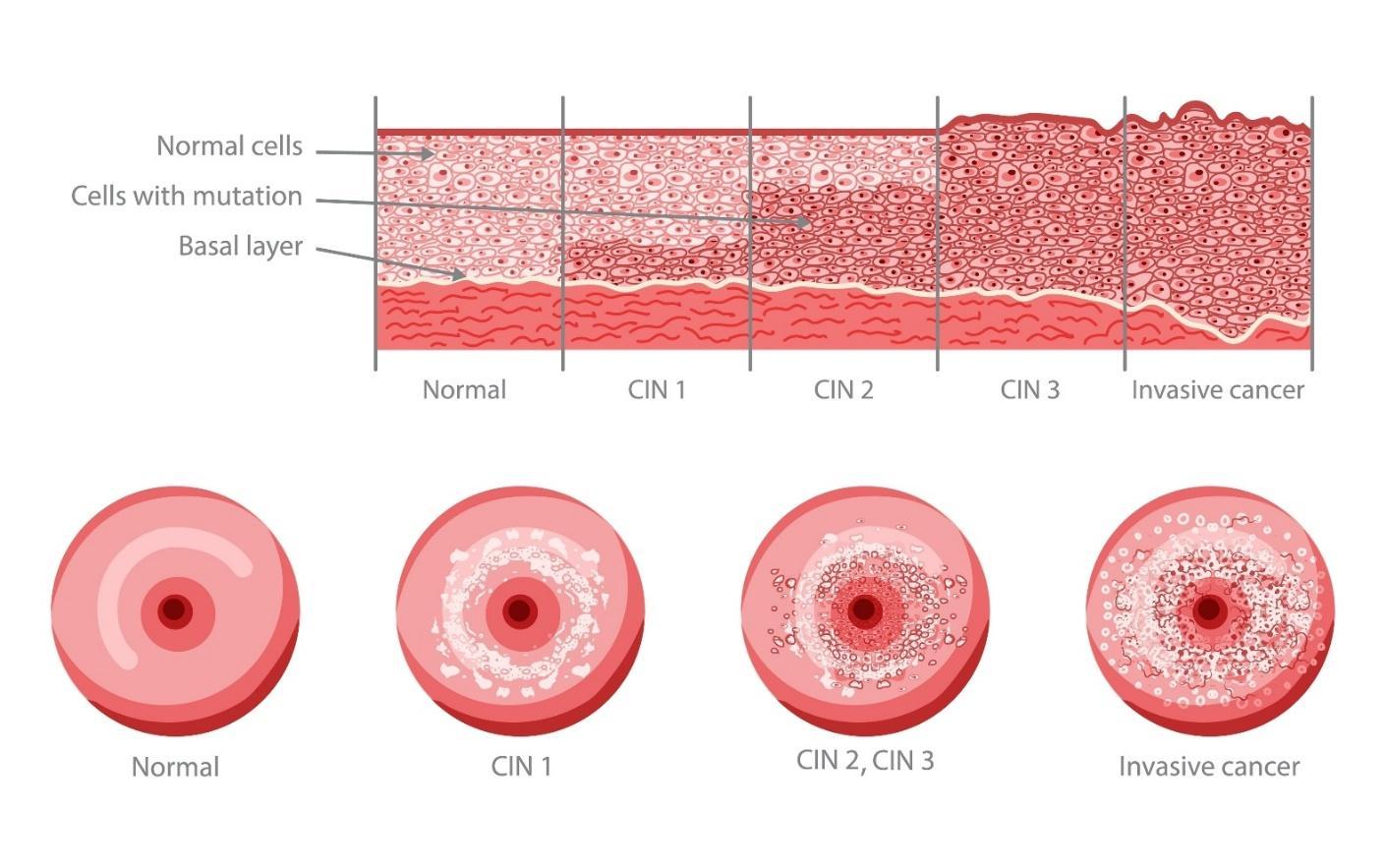
Founded in 2022, Amplexd Therapeutics has been gaining prominence in the women’s health space as a company at the technological vanguard of the treatment of CIN, a disease which affects upwards of 178 million women globally every year. About 5% of cervical screenings in the US and Europe reveal abnormalities at any given time, with significantly higher rates in Asia, Latin America, and Africa. CIN results from certain oncogenic varieties of HPV, which can transform cervical cells into invasive cancer if left untreated. Cervical cancer is the fourth leading cause of cancer death globally in women and can largely be prevented by treatment at the CIN stage.1
Amplexd was born from Rahman’s personal experience with persistent CIN. Following a diagnosis of carcinoma in situ (high-grade CIN) and an invasive surgery, a 13-year journey with chronic CIN ensued. Eager to avoid a subsequent surgery, Rahman set out to develop a non-invasive topical therapy using a primary active ingredient, Epigallocatechin gallate (EGCG), a polyphenol derived from green tea. Evidence for the efficacy of green tea polyphenols against CIN had been investigated in a research setting but hadn’t been adequately developed from the perspective of becoming a commercial therapeutic. Seizing upon this gap, Amplexd Therapeutics was born. “I envisioned the advent of an alternative to ‘watching and waiting’ and surgery for this incredibly common condition, having myself experienced the psychological burden of chronic CIN, the trauma of the surgery itself, and the revolving door of doctors’ appointments. Our non-invasive treatments have the potential to be a game-changer both in terms of access and comfort, as they wouldn’t require surgery or physicians to implement, and are designed to minimize discomfort. My hope is that once approved, others can have an experience better than my own, without compromising quality of care,” Rahman added.
About Amplexd Therapeutics, Inc.
Amplexd Therapeutics is developing two low-cost, novel, non-invasive therapies for the treatment of Cervical Intraepithelial Neoplasia (cervical precancer). The first is an intravaginal suppository for low-grade CIN, and the second is a photodynamic therapy (PDT) system for high-grade CIN, each designed to selectively target neoplastic cells. These treatments are designed to both modernize the paradigm for women in developed nations by sparing the need for invasive surgeries, as well as provide access to potentially lifesaving care for those in low and middle-income regions.
For more information about Amplexd Therapeutics, Inc., please visit amplexd.com.
Contact:
Media Relations - Amplexd Therapeutics, Inc., Email: [email protected]
Forward-Looking Statement:
This press release may contain forward-looking statements that are subject to risks and uncertainties. Actual results may differ materially from those expressed or implied in such statements. Factors that could cause actual results to differ include, but are not limited to, the regulatory and commercial success of Amplexd Therapeutics, as well as the competitive landscape in the women’s health, infectious disease, and oncology markets. Amplexd Therapeutics, Inc. assumes no obligation to update any forward-looking statements.
1 World Health Organization. https://www.who.int/news-room/fact-sheets/detail/cervical-cancer Accessed October 4, 2023.



