Colorectal cancer (CRC) is a highly malignant disease that readily metastasizes to vital organs. Many strategies have been employed for clinical CRC therapy. However, current treatments face significant limitations, such as drug toxicity, tumor recurrence, and drug resistance due to gene mutations in CRC cells. Therefore, there is an urgent need to develop novel anti-tumor drugs that can effectively treat CRC patients with diverse genetic profiles.
This research, published in the Genes & Diseases journal by a team from the Sun Yat-sen University Cancer Center, Sun Yat-sen Memorial Hospital, Affiliated Hospital of Jiangnan University, and Southern Medical University, focuses on a third-generation photosensitizer, Ce6-GFFY. The researchers developed this novel molecule by covalently combining a photo-responsive Ce6 molecule with a GFFY peptide. Ce6-GFFY forms stable macroparticles with an average size of 160 nm in solution, enabling targeted tumor penetration through the enhanced permeability and retention (EPR) effect.
In the in vitro studies, Ce6-GFFY demonstrated effective penetration and induced significant ROS production in CRC cells upon irradiation with a 660 nm laser. Furthermore, results showed that CRT, a classical hallmark that acts as an “eat-me” signal, is highly expressed in Ce6-GFFY-treated CRC cells. This suggests that Ce6-GFFY can effectively induce immunogenic cell death (ICD), indicating its promising potential for CRC therapy.
In the in vivo studies, the macroparticles exhibited a prolonged half-life in mice, demonstrating effective drug uptake and an extended therapeutic window. The combined use of Ce6-GFFY and laser irradiation significantly activated anti-tumor immunity by promoting cytotoxic T cell infiltration while inhibiting the accumulation of myeloid-derived suppressor cells in tumors, thereby suppressing the growth of both primary and metastatic CRCs. These findings suggest that Ce6-GFFY is a promising agent for CRC therapy with minimal side effects.
Unlike traditional treatments, photodynamic therapy (PDT) with Ce6-GFFY can destroy cancer cells regardless of genetic mutations, making it a versatile therapy option for patients who cannot be treated with any existing therapeutics, including those with clinical drug resistance. In conclusion, the researchers highlight that the development of Ce6-GFFY represents a promising new strategy for the treatment of colorectal cancer.
https://tinyurl.com/mtcjnvtb
Reference
| Title of Original Paper | Ce6-GFFY is a novel photosensitizer for colorectal cancer therapy |
|---|---|
| DOI | https://doi.org/10.1016/j.gendis.2024.101441 |
| Journal | Genes & Diseases Genes & Diseases is a journal for molecular and translational medicine. The journal primarily focuses on publishing investigations on the molecular bases and experimental therapeutics of human diseases. Publication formats include full length research article, review article, short communication, correspondence, perspectives, commentary, views on news, and research watch. |
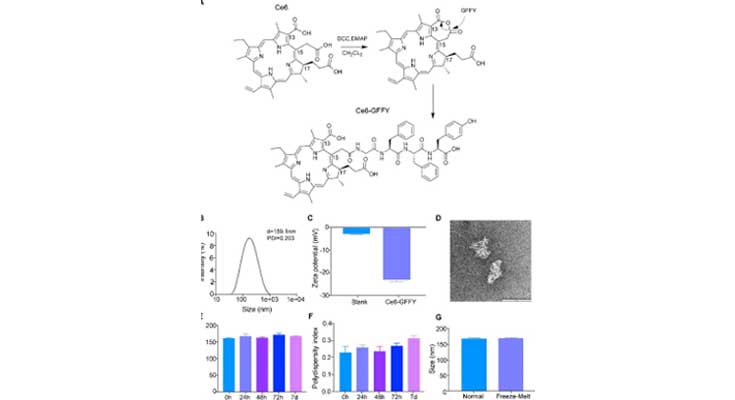
Click to enlarge
Image Title: Synthesis and characterization of Ce6-GFFY.
Image Caption: (A) Schematic diagram of Ce6-GFFY synthesis. DCC, N, N0-dicyclohexylcarbodiimide; DMAP, 4-dimethylaminopyridine; CH2CL2, dichloromethane. (B) The size distribution of Ce6-GFFY macroparticles was analyzed using DLS. d, diameter; PDI, polydispersity index; DLS, dynamic light scattering. The data are representative of five independent experiments. (C) The Zeta potential of Ce6-GFFY macroparticles was analyzed using DLS. Blank, PBS. The data are representative of five independent experiments. (D) Ce6-GFFY macroparticle image was photographed by transmission electron microscopy. Scar bar, 100 nm. (E, F) Particle size (E) and polydispersity index (F) of Ce6-GFFY macroparticles incubated at 37 °C at different times as indicated was detected by DLS. The data are representative of five independent experiments. (G) Particle size of Ce6-GFFY incubated at room temperature (normal) or underwent - 80 °C /37 °C freezing-thawing (Freeze-Melt) was detected by DLS. The data are representative of five independent experiments.
Image credit: The authors
Image link: https://ars.els-cdn.com/content/image/1-s2.0-S2352304224002381-gr1_lrg.jpg
License type: CC BY-NC-ND
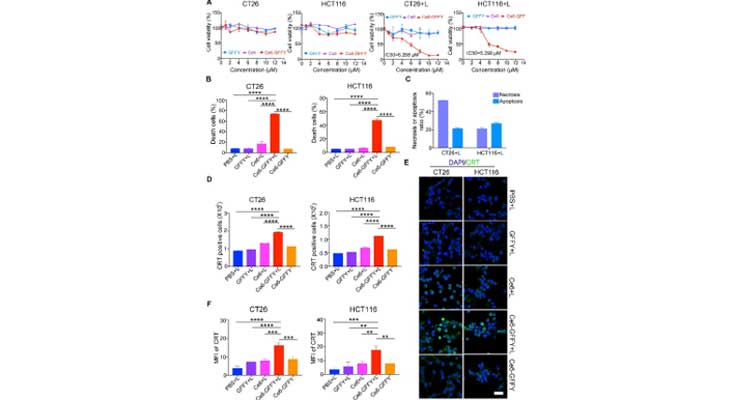
Click to enlarge
Image Title: Ce6-GFFY suppresses the proliferation of colorectal cancer cells.
Image Caption: CT26 and HCT116 cells were treated with indicated agents at 37 °C for 1 h and then treated with or without 660 nm laser irradiation for 1 min at a power of 0.02 W/cm2. The data are representative of three independent experiments. (A) Cells were incubated at 37 °C for 24 h after being treated with laser irradiation and the indicated dose of GFFY peptide, Ce6, and Ce6-GFFY, and then cell proliferation was determined using CCK-8 assays. The IC50 of Ce6-GFFY under laser irradiation was analyzed. IC50, 50 % inhibitory concentration. (B) Cells (CT26, 10 μM; HCT116, 5 μM) were stained with annexin V and propidium iodine dye, and then the ratio of dead cells was analyzed using flow cytometry. (C) The ratio of necrotic and apoptotic cells in the combined treatment of Ce6-GFFY and laser irradiation were analyzed. (D–F) After treated with the indicated agents (CT26, 10 μM; HCT116, 5 μM), cells were incubated at 37 °C for 8 h and stained with a CRT antibody, then the expression of CRT was analyzed using flow cytometry (D) and confocal laser scanning microscopy (E), and the CRT fluorescence was analyzed (F). CRT, calreticulin; green, CRT; blue, DAPI; MFI, mean fluorescence intensity. Scale bar, 40 μm “L” in “CT26+L”, “HCT116+L”, “PBS + L”, “GFFY + L”, “Ce6+L”, “Ce6-GFFY + L”: laser irradiation. Statistical analyses were performed using one-way ANOVA; bars, standard deviation; ∗∗P < 0.01; ∗∗∗P < 0.001; ∗∗∗∗P < 0.0001.
Image credit: The authors
Image link: https://ars.els-cdn.com/content/image/1-s2.0-S2352304224002381-gr3_lrg.jpg
License type: CC BY-NC-ND
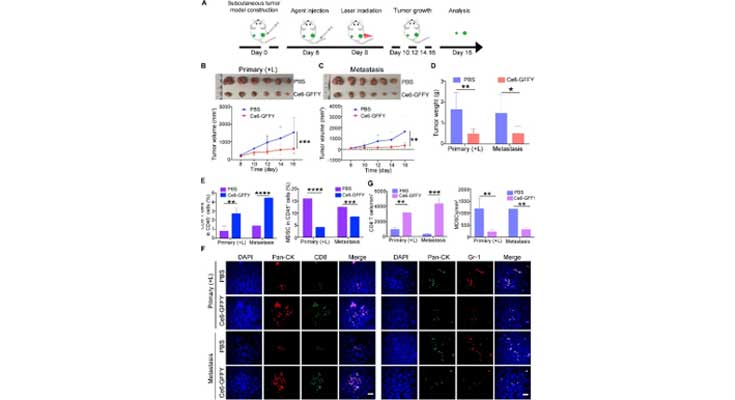
Click to enlarge
Image Title: Ce6-GFFY activates anti-tumor immunity and suppresses metastatic tumor growth.
Image Caption: Primary and metastasis tumor model was constructed by subcutaneously transplanting CT26 cells in the left (metastasis tumor) and right flanks (primary tumor) of BALB/c mouse. Then Ce6-GFFY (5 mg/kg) was injected through the tail vein of the mouse, and the 660 nm, 0.2 W/cm2 laser irradiation (1 min on, 1 min off; 4 cycles) was performed on the primary tumor 6 h after the injection. Only a single dose was administered during the entire treatment cycle. (A) Schematic diagram of the mouse model construction and PDT strategy. (B) Primary tumor tissues were collected and tumor growth was analyzed after treatment. n = 6. (C) Metastasis tumor tissues were collected and tumor growth was analyzed after treatment. n = 6. (D) Primary and metastasis tumors were weighed and analyzed. n = 6. (E) Primary and metastasis tumors were collected and dispersed into single cells for flow cytometry analysis, and the amount of cytotoxic T cells (CD45+CD3+CD8+) and myeloid-derived suppressor cells (MDSCs, CD45+CD11b+Gr-1+) were analyzed. n = 3. (F, G) IF staining for CD8+ T cells (CD8) and MDSCs (Gr-1) in primary and metastasis tumors (F), and the number of positive cells per mm2 was analyzed (G). n = 3. IF, immunofluorescence. “(L)” in “Primary (+L)”: laser irradiation. Scale bar, 20 μm. The data are representative of three independent experiments. Statistical analyses were performed using unpaired t-test; bars, standard deviation; ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; ∗∗∗∗P < 0.0001.
Image credit: The authors
Image link: https://ars.els-cdn.com/content/image/1-s2.0-S2352304224002381-gr6_lrg.jpg
License type: CC BY-NC-ND
Funding Information:
The National Natural Science Foundation of China (No. 82373174, 82002466, 82202907)
Genes & Diseases publishes rigorously peer-reviewed and high quality original articles and authoritative reviews that focus on the molecular bases of human diseases. Emphasis is placed on hypothesis-driven, mechanistic studies relevant to pathogenesis and/or experimental therapeutics of human diseases. The journal has worldwide authorship, and a broad scope in basic and translational biomedical research of molecular biology, molecular genetics, and cell biology, including but not limited to cell proliferation and apoptosis, signal transduction, stem cell biology, developmental biology, gene regulation and epigenetics, cancer biology, immunity and infection, neuroscience, disease-specific animal models, gene and cell-based therapies, and regenerative medicine.
Scopus CiteScore: 7.3
Impact Factor: 6.9
More information: https://www.keaipublishing.com/en/journals/genes-and-diseases/
Editorial Board: https://www.keaipublishing.com/en/journals/genes-and-diseases/editorial-board/
All issues and articles in press are available online in ScienceDirect (https://www.sciencedirect.com/journal/genes-and-diseases).
Submissions to Genes & Disease may be made using Editorial Manager (https://www.editorialmanager.com/gendis/default.aspx ).
Print ISSN: 2352-4820
eISSN: 2352-3042
CN: 50-1221/R
Contact Us: [email protected]
X (formerly Twitter): @GenesNDiseases (https://x.com/GenesNDiseases)
 MEDINDIA
MEDINDIA