- Hunter LE, Simpson JM. Prenatal screening for structural congenital heart disease. Nat Rev Cardiol. 2014 Jun;11(6):323-34.
- Dodge-Khatami A. Advances and research in congenital heart disease. Translational Pediatrics. 2016;5(3):109-111.
- Congenital Heart Defects - (https://medlineplus.gov/congenitalheartdefects.html)
- About Congenital Heart Defects - (http://www.heart.org/heartorg/conditions/congenitalheartdefects/congenital-heart-defects_ucm_001090_subhomepage.jsp)
- What is Congenital heart defect? - (https://en.wikipedia.org/wiki/congenital_heart_defect)
- What Are Congenital Heart Defects? - (https://www.nhlbi.nih.gov/health/health-topics/topics/chd)
- Congenital heart defects and CCHD - (http://www.marchofdimes.org/complications/congenital-heart-defects.aspx#)
What are Congenital Heart Defects?
Congenital heart defects (CHD) are abnormalities in the structure of the heart that affects the way the heart functions. CHDs usually develop during the first few weeks of pregnancy.
CHDs affect the blood flow through the heart and to the rest of the body. The defects range from mild defects that need no treatment or those that can be easily fixed, to severe, life threatening defects that require special medical and surgical care soon after birth. Twenty five percent of CHDs come under the critical category, also known as critical congenital heart defects (CCHD).
CCHDs are termed critical because they cause inadequate oxygenation of blood. This is because the blood from the right and left sides of the heart get mixed up. This leads to cyanosis (bluish discoloration of skin and mucous membranes). Hence a broader or alternate way to categorize CHDs are defects that can be “Cyanotic” or “Acyanotic”.
Critical defects need surgery within the first year of the baby’s life to get corrected. Nowadays, due to advancements in diagnosis and treatment methods, even children with complex heart defects survive into adulthood. They are capable of living active, productive lives.
About the Heart
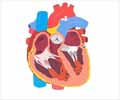
The heart consists of two upper chambers called the atria and two lower chambers called the ventricles. The heart is also divided into left and right portions with a septal wall in between. The chambers on the heart’s left are the left atrium and left ventricle while the ones on the heart’s right are the right atrium and the right ventricle.
At the start of each heartbeat, blood fills up both the atria. The oxygen-poor blood returns from the body into the right atrium via the superior and inferior vena cavae, and the oxygen-rich blood brought from the lungs via the pulmonary veins fills up the left atrium.
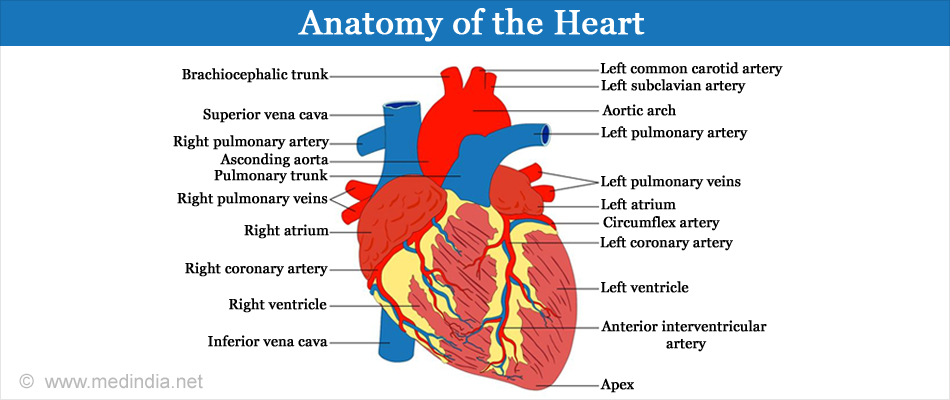
The oxygen-poor blood from the right atrium goes to the right ventricle through the tricuspid valve. From here, the blood goes to the lungs via the pulmonary artery to get rid of the carbon dioxide and get filled with oxygen and returns to the left atrium of the heart via the pulmonary veins. At the origin of the pulmonary artery is the pulmonary valve.
The oxygen-rich blood from the left atrium goes to the left ventricle through the mitral or bicuspid valve. From here, the blood leaves the heart via the aorta, the largest artery of the body to circulate oxygen-rich blood to the rest of the body. At the origin of the aorta at the heart is the aortic valve.
The valves make sure that the blood flows in a single direction and the septum prevents mixing of the blood between the two sides.
Facts about Congenital Heart Defects
- Heart defects are the most common type of birth defects occurring in 1% of live births in the United States.
- The year 2013 reported 34.3 million people having a congenital heart disease.
- The risk for a second offspring of being born with a heart defect in a family with no history of the heart disease in the family is 3-5%.
- More than 35,000 babies are born with congenital heart defects each year in the United States.
- More than 1 million adults are living with congenital heart defects in the United States.
What are the Causes of Congenital Heart Defects?
In most cases, no known cause for CHD can be detected.
Genetic components like chromosomal abnormalities and single gene defects combined with environmental factors like the mother’s diet could be causative or risk factors involved.
Medications like the anti-seizure pills containing valproate, lithium, thalidomide and the acne medication isotretinoin - The doctor should be aware if these medicines are being taken before pregnancy so he can prescribe substitutes for them to be taken during pregnancy.
Contracting rubella or German measles during early pregnancy - The doctors can test the mother’s immunity against this viral infection and immunize her before pregnancy if necessary.
Having insulin-dependent diabetes, phenylketonuria, deficiency of B vitamin folic acid and not adhering to recommended diet during pregnancy are risk factors. Controlling sugar levels by diabetes patients before and during pregnancy lowers the risk factor. Consuming 400 micrograms of folic acid daily before and during early pregnancy can help in the prevention of CHDs.
Smoking and consuming alcohol are to be avoided during pregnancy.
There is an 8-10 % risk that the child might inherit a heart defect if the mother has a CHD herself (genetic). Fifteen percent of all CHDs are associated with genetic conditions like Down’s syndrome (caused by an extra 21st chromosome) and DiGeorge syndrome (caused by deletion of chromosome number 22). If the older child has been born with a heart defect, a genetic counselor can estimate the odds for the next child developing the heart disease by genetic testing.
What are the Symptoms and Signs of Congenital Heart Defects?
Depending on the type and severity of the condition, symptoms and signs can be:
- A heart murmur (an extra or an unusual sound that is heard during a heartbeat through the stethoscope)
- Rapid breathing that is faster than normal
- Shortness of breath
- Poor feeding
- Poor growth
- Tiring easily while feeding
- Sweating with exertion
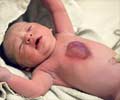
- Bluish skin, nails or lips - cyanosis
- Lung infections
How are Birth Defects Diagnosed?
Severe congenital heart defects can be detected during pregnancy, at birth or during infancy. Less severe defects might not get diagnosed till childhood or even adulthood; sometimes, they are detected by chance based on results from a physical exam and tests done for another reason.
Prenatal screening tests are offered to women during pregnancy to check if the
baby might have some problem. These do not provide specific diagnosis.
- An ultrasound completed between 11 and 13 weeks looks for certain birth defects related to the heart. It takes pictures and looks for extra fluid at the back of the baby’s neck. An increase in the amount of fluid would indicate a heart defect.
- If the results from the screening test indicate a congenital heart defect or a risk for it, a specific diagnosis can be done with a fetal echocardiogram at about 18 to 22 weeks of pregnancy. The test creates detailed ultrasound pictures of the baby's heart and shows problems with the heart’s structure and its functioning capacity.
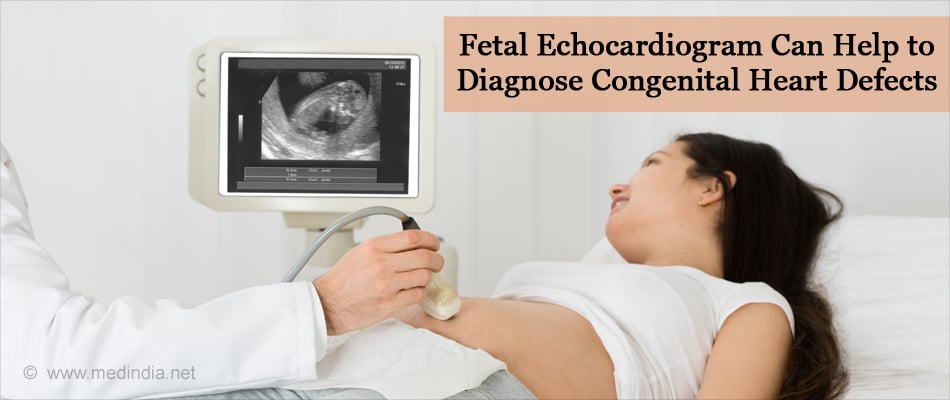
Early diagnosis of a congenital heart defect helps the doctor plan the required treatments before the baby is born and gives the parents time to be informed about the prognosis of the fetus. If the natural history suggests an unfavorable outcome, a fetal cardiac intervention is an option.
Parameters like operator expertise, gestational age, fetal position and the type of cardiac defect affect the sensitivity of CHD detection. Many newborns with simple CHD and some newborns with CCHD may be missed due to this.
Post delivery:
After birth, an abnormal heart sound or a heart murmur is the first sign of a heart defect.
For a significant heart problem, the child is referred to a pediatric cardiologist who is trained to diagnose and treat heart problems in infants, children and young adults. They can tell what tests, medications, interventional procedures and surgeries are needed, and the frequency of heart checkups needed in the future.
Physical examination of the child is done where the doctor will -
- Look for any symptoms like rapid breathing, shortness of breath or cyanosis. The child will be monitored in the first few visits for delayed growth.
- Look for any abnormalities in the child’s heartbeat or lungs with a stethoscope at birth and during the follow-up visits.
If any abnormalities are noticed by the doctor, the following diagnostic tests can be done to detect the exact defect.
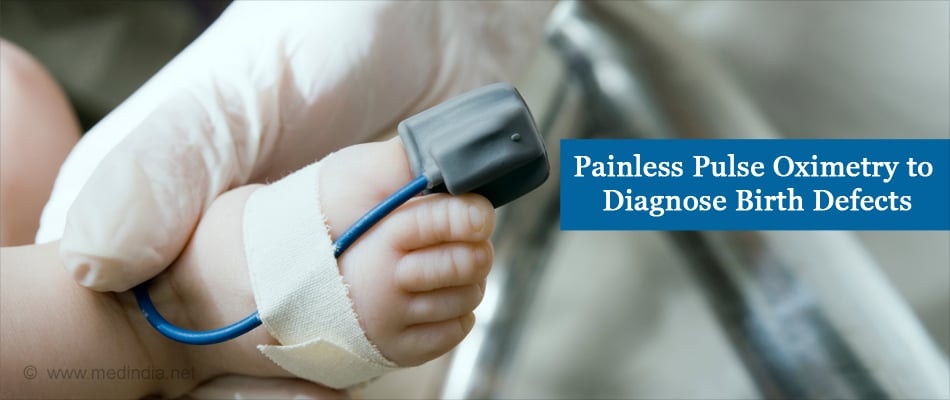
Pulse oximetry is a painless, noninvasive examination which uses a small sensor that is clipped to the baby’s finger or toe. It measures the percentage of hemoglobin in the blood that is saturated with oxygen and the pulse rate.
Echocardiography: Using ultrasound waves to capture moving pictures of the structures of the heart this important test reveals whether the heart
- Is structurally normal or not
- Is functioning
- Is reacting to these problems
- Can be treated if and when required
An electrocardiogram records the heart’s electrical activity and checks
- The rate and rhythm of heart beat
- The strength and timing of electrical signals that pass through the heart
- If the chambers are enlarged
A chest x-ray takes pictures of the heart and lungs and can detect
- If the heart is enlarged
- Extra blood flow or extra fluid in lungs
For a detailed diagnosis more tests are performed:
In cardiac catheterization a contrast agent is sent into a blood vessel or one of the heart's chambers through a thin, flexible tube called a catheter; the direction of blood flowing through the heart is then seen on an x-ray image. This device
- Measures the pressure and oxygen level in the blood vessels and chambers
- Checks if the blood is getting mixed between the two sides of the heart
As a treatment procedure, devices can be attached to the catheter to repair the heart defect.
Magnetic resonance imaging that uses painless magnet waves and computerized tomography that uses multiple x-ray images can also be done to take clearer pictures of the heart and lungs.
Trans-esophageal echocardiogram uses a special long tube with a small ultrasound probe at the end, called a TEE probe while the patient is sedated. It takes an ultrasound movie of the heart and is recommended
- When pictures from a standard echocardiogram are not clear enough
- To provide more information to guide treatment if a heart surgery has to be performed
- To help the surgical team to assess the success of the procedure
Holter monitor is a small recorder attached to the body that records every heart beat for 24 hours. Events like awake time, active time, sleep time and any symptoms that can occur have to be recorded in a diary.
The information from the recorder is processed by a technician and a cardiologist will review it.
Since the recording duration of a Holter monitor is 24 hours, it can detect a dangerous heart rhythm and even symptoms that occur only once a day can be tracked. If symptoms occur less frequently, alternatively the doctor would prescribe an event monitor.
What are the Types and Treatments of Congenital Heart Defects?
1. Common or acyanotic congenital heart defects:
A. Holes in the heart or septal defects:
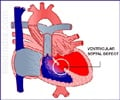
A hole in the septum between the atria is called an atrial septal defect (ASD) and hence causes mixing of blood between the two atria.
Treatment - The amount of blood that gets leaked depends on the size of the hole. Small holes can close on their own with age. Middle-sized and large holes might need repair with a catheter insertion or an open heart surgery.
The catheter that is threaded to the heart’s septum is attached with two small disks or an umbrella-like device that gets pushed out of the catheter on reaching the septum. This device plugs the hole between the atria and the catheter is then removed from the body. Normal tissue grows over the device covering it in about 6 months.
A hole in the septum between the ventricles is called a ventricular septal defect (VSD). The oxygen-rich blood in the left ventricle that should flow out of the aorta, then flows into the right ventricle.
Treatment - Small-to-medium holes can close on their own with age. Large holes leak a lot of blood from the left to the right ventricle forcing the left ventricle to work harder to push blood through the aorta. More blood in the right ventricle causes high blood pressure in the heart and the lung arteries. This load on the heart can lead to heart failure. Open heart surgeries can be performed to close the VSD.
B. Patent ductus arteriosus (PDA):
The ductus arteriosus is the blood vessel connecting the pulmonary and the aortic arteries in the fetal blood circulation. The ductus closes within a few minutes to a few days after the baby is born. If it fails to and remains open or patent, oxygen-rich blood from the aorta mixes with the oxygen-poor blood from the pulmonary artery increasing the strain on the heart and the pressure in the lung arteries.
Treatment - Small PDAs can close on their own. For larger ones, catheter-based procedures or surgeries might be needed.
C. Valve defects:
Defects of the valves manifest as the following:
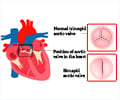
- The valve’s flaps are thick or fused together and the valve cannot open fully thus causing narrowing and obstruction - stenosis.
- The valve is not formed properly and there is no hole for the blood to pass through - atresia.
- The valve does not close tightly and there is a leak backwards and this causes insufficiency - regurgitation.
The most common valve defect is pulmonary artery stenosis that affects the pulmonary valve. As a result the right ventricle gets worked up and has to push hard to get the blood into the pulmonary artery.
If this condition occurs along with a PDA or an ASD, the oxygen-poor blood from the right side gets mixed with the blood in the left side. As a result of low oxygen blood flowing out of the aorta, cyanosis can occur.
Treatment - Mild pulmonary artery stenosis might nor require treatment while a severe case might be corrected with a catheter-based procedure where a tiny balloon is attached to the end of the catheter and inflated to push apart the valve’s flaps. The balloon is then deflated and both the catheter and the balloon are removed from the body. This procedure can be used to treat any narrowed heart valve.
In aortic valve stenosis, the defective valve is the aortic valve. The left ventricle has to work hard to pump the normal amount of blood out to the body plus the surplus blood that gets regurgitated back into the chamber due to the defective valve. As a result, the left ventricle can get enlarged and there is thickening or hypertrophy of the heart muscle; over time, the heart muscle can get damaged.
D. Coarctation of the aorta (CoA):
In this condition, the aorta is narrowed and constricted, obstructing blood flow from the heart mainly to the lower part of the body. The blood pressure in the left ventricle above the point of constriction increases forcing the heart to push harder to get the blood out of the heart. Coarctation of the aorta can cause thickening and damage to the overworked heart muscle.
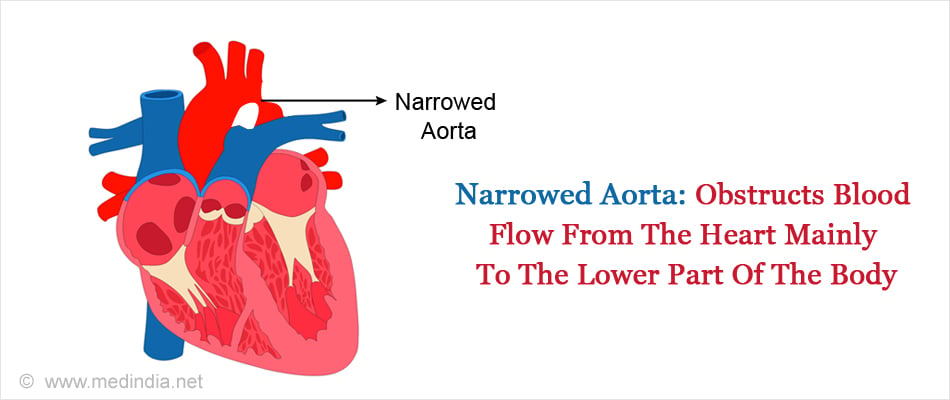
Treatment - Coarctation may be corrected by catheterization or surgery. During catheterization, a balloon is inflated at the narrowed site and a stent is placed to keep the part open. In a surgery, the narrowed segment of the aorta can be removed or a patch can be sewed over the narrowed section using a component of a blood vessel of the arm or a graft of a synthetic material.
2. Critical or cyanotic congenital heart defects:
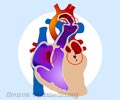
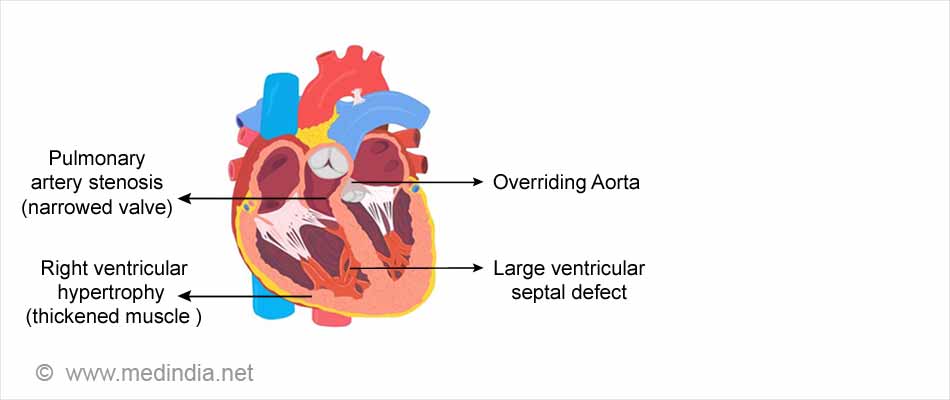
A. A common complex heart defect is Tetralogy of Fallot that is so named because it comprises of four simultaneous heart defects that affects the blood flow through the heart.
The four defects are -
- Pulmonary artery stenosis
- A large ventricular septal defect
- An overriding aorta that is located directly over the VSD between the left and right ventricles. Oxygen-poor blood finds its way into the aorta instead of into the pulmonary artery.
- Right ventricular hypertrophy where the right ventricle muscle becomes thicker due to overworking
Temporary treatment involves placing a shunt (small tube) of a synthetic material in between the aorta or other body artery and the pulmonary artery so as to provide enough blood to flow to the lungs to get oxygenated.
Definitive treatment also aims to increase the right ventricular outflow to the lungs by
- Removing thickened muscle under the pulmonary valve
- Removing or repairing the pulmonary valve
- Enlarging the branch arteries that lead to the lungs along with closing the VSD.
B. Transposition of the great arteries:
The normal blood flow pattern in the body is body-heart-lungs-heart-body.
In d-transposition, the two main arteries carrying blood away from the heart are switched leading to the blood flow getting stuck in 2 pathways, namely
- Body-heart-body where the blood does not go to the lungs to get oxygen.
- Lungs-heart-lungs where the oxygen is not delivered to the body.
Treatment - The infant is catheterized to buy time before a corrective surgery can be done; catheterization involves enlarging connections between the atria.
Surgery for d-transposition is of two types. The first procedure, the Mustard procedure or the Senning procedure involves an atrial switch where a tunnel or a baffle is created between the atria. The oxygen-rich blood is redirected to the right ventricle and aorta and the oxygen-poor blood is redirected to the left ventricle and the pulmonary artery.
The second and preferred procedure is called the arterial switch operation in which, the aorta and pulmonary artery are switched back to their normal positions. The coronary arteries carrying the oxygen-rich blood to the heart muscles are re-attached to the new aorta.
In l-transposition, the right and left ventricles are switched, and as a result, the great arteries are also switched. This condition is less dangerous since this ‘double reversal’ ensures that the body still receives oxygen-rich blood and the lungs still get the oxygen-poor blood.
Although the systemic and the pulmonary circulations are connected, in the long run, complications can arise because the right atrium is not designed to pump blood at a high pressure against the body circulation.
Treatment - If there is no VSD or pulmonary valve obstruction, surgery might not be needed. If they are present, the repair should be performed first and a “double switch” operation performed to reroute the oxygenated blood to the left ventricle and the oxygen-poor blood to the right ventricle.
C. Total Anomalous Pulmonary Venous Connection (TAPVC):
The veins coming from the lungs to the heart are attached to the heart in abnormal positions. Hence, the oxygen-rich blood from the pulmonary veins enters or leaks into the wrong chamber of the heart, the right atrium where it gets mixed with oxygen-poor blood. This blood can then flow through an ASD to the left atrium, then the left ventricle and out to the body. Since this mixed blood is now-oxygen-poor, the child looks blue.
Treatment must be a surgery in early infancy. An open-heart surgery connects the pulmonary veins to the left atrium and closes the ASD.
D. Hypoplastic left heart syndrome
As the name suggests, the parts of the left side of the heart including the aorta, aortic valve, left ventricle and the mitral valve are underdeveloped. The heart of babies with this defect compromise by allowing the blood to flow through the atrial septal defect between the atria when it returns from the lungs. The right ventricle pumps the blood into the pulmonary artery that reaches the aorta through the patent ductus arteriosus.
Treatment is a series of operations or a heart transplantation. Until then, the ductus is kept open with intravenous medications.
Surgery is done in several stages -
The first stage is called the Norwood procedure that has to be performed right after birth. It involves directing the blood to the lungs through a shunt so that the right ventricle can pump the blood to both the body and the lungs without the need for the ductus to be open.
The second stage (bidirectional Glenn or hemi-Fontan) performed between 4 and 12 months and the third stage (lateral tunnel Fontan or extracardiac Fontan) done between 18 months and 3 years and maybe some minor operations in between connect the veins returning oxygen-poor blood to the heart and the pulmonary artery. The final aim is to make the right ventricle pump only oxygenated blood to the body so that cyanosis reduces or does not occur.
Heart transplantation might be recommended as an alternative. It provides the infant with a normal heart but along with a life-long dose of medications to prevent rejection and the development of transplant-related problems.
E. Pulmonary Atresia:
This is a valve defect where the pulmonary valve is absent and the tricuspid valve and the right ventricle are poorly developed. Blood is unable to flow from the right ventricle to the lungs to get oxygenated. The supply of lung blood is acquired through the PDA.
An atrial septal opening helps by letting the oxygen-poor blood from the right atrium mix with the oxygen-rich blood of the left atrium that has come from the lungs. Then this mixture of partially oxygenated blood is delivered to the body making the infant appear bluish or cyanotic.
Treatment of pulmonary atresia with intact ventricular septum:
Temporary treatment -
- A drug can be given to keep the PDA from closing.
- A shunt can be created between the aorta and the pulmonary artery to increase the blood flow to the lungs.
Permanent treatment in the form of a surgery (Glenn and Fontan procedures) depends on
- The size of the pulmonary artery and the right ventricle
- Sinusoids or abnormal channels that form between the coronary arteries and the right ventricle
Open heart surgery is helpful only if the pulmonary artery and the right ventricle are somewhat normal sized. If the right ventricle is very small to be a good pumping chamber, the body veins are connected directly to the pulmonary arteries and the atrial defect is closed.
Tricuspid Atresia:
This is a valve defect where the tricuspid valve is absent and the right ventricle is small and poorly developed. Blood is unable to flow from the right atrium to the right ventricle.
An atrial septal opening helps by letting the oxygen-poor blood from the right atrium mix with the oxygen-rich blood of the left atrium that has come from the lungs. A ventricular septal opening is also present. Then most of this mixture of partially oxygenated blood is delivered from the left ventricle via the aorta to the body making the infant appear bluish or cyanotic. Some of it flows through the VSD into the small right ventricle back to the lungs.
Treatment -
A surgical procedure is performed to place a shunt to increase blood flow to the lungs to decrease cyanosis.
For children who have too much blood flowing to the lungs, a corrective surgery named pulmonary artery banding is done to decrease the flow and to protect the lung blood vessels.
Complete repair can be done on babies to make their hearts work normally and route the oxygen-poor blood from the lower half of the body and liver to the lungs.
Two opeartions, a bidirectional Glenn operation where the superior vena cava (the large vein that brings blood from the upper part of the body to the heart) and a Fontan operation where the inferior vena cava (the large vein that brings blood from the lower part of the body to the heart) are connected to the pulmonary arteries. This procedure helps to improve the cyanosis better if the right ventricle is of a nearly normal size.
With medical advancements in today’s age, a child born with a congenital heart defect can get the best care and treatment to lead a normal life.