About
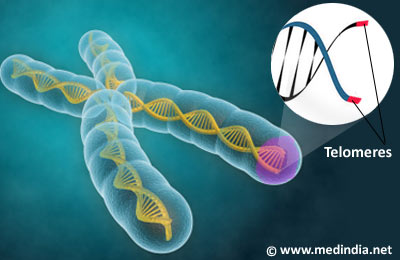
Telomeres are cap-like structures found at the end of the chromosomes consisting of tandem repeats of DNA that does not encode for any gene product, but protects the end of the chromosome from deterioration and fusion with neighboring chromosomes.
The role of this ‘junk’ DNA is to protect the ‘real’ DNA from the effects of cell division.
The telomeres help distinguish between the DNA ends and the breaks that occur within the DNA. When the DNA within the chromosome breaks, it triggers the salvaging mechanisms such as DNA repair, or even apoptosis (programmed cell death). The DNA ends must not be mistaken for breaks which prompt repair measures, and this is achieved by the telomeres which cap the ends.
Telomere and Senescence (Biological Ageing)
DNA polymerase is the enzyme that is responsible for DNA replication. Unfortunately this enzyme is incapable of recognizing the ends of linear DNA during the S-phase of cell division, resulting in the shortening of telomeres. Other factors too contribute towards telomere shortening, such as suppression of telomerase activity and reactive oxygen species (ROS).
The telomeres become shorter with each cell division and lose their capping function. Eventually the DNA cannot be copied anymore and the cell reaches senescence or cell death.
Telomere shortening also acts as a tumor suppressor mechanism but on the downside it is the hall mark of impaired organ maintenance and cell dysfunction.
Experiments in telomerase-deficient mice have revealed that a shortening of telomerase is associated with organ maintenance and life span.
Cells in many of the organs show telomere shortening including that of the kidney, lungs, intestine, hepatocytes, muscles and vascular tissues. However, the telomeres in the brain cells do not exhibit shortening, except for the blood cells in Alzheimer patients which show telomere reduction.
Similarly, skin cells also do not show marked telomere shortening although senescence is observed in ageing skin cells. In population level studies, research has shown that older individuals have shorter telomeres. Most cells in humans can replicate approximately 50 times before the telomeres become too short to divide any longer. To counter this reduction in telomere length an enzyme exists, known as telomerase, which is capable of synthesizing telomeres de novo. This enzyme is very active during embryogenesis but is suppressed soon after birth, in humans. However it is reactivated in the germ cells, some stem cells and other progenitor cells. That is why many believe that telomeres hold the “secret to longevity.”
Although telomere shortening is reduced in stem cells, the level of telomerase in stem cells is not sufficient to support a life time of stem cell activity and sustenance, proving that telomere shortening is linked to ageing.
Telomere Shortening and Diseases
Telomere shortening is not just a feature of ageing but is also closely linked to diseases.
Shortening of telomeres is observed in cases of chronic HIV, chronic hepatitis, inflammatory bowel diseases, various forms of anemia, artherosclerosis and liver cirrhosis. A classical association of telomere shortening and disease progression is seen in
Cancer is a disease that comes with old age. Cancer cells are characterized by uncontrolled division and it is of great significance to note that the telomerase gets activated in 90% of cancer patients. Contradictorily, the telomeres get shortened in individuals with cancer. Telomere length and telomerase activity has been considered as markers for disease initiation and progression in the case of various tumors and cancers such as those of the liver, lung, breast prostate, brain head and neck, pancreas and hematopoietic system.
Researchers in Sweden have revealed that the telomeres in some people do not reduce with time and that it even gets longer in some!! There exist individual variations that are not easy to explain. Some scientists believed that those with telomeres that do not shorten easily may have a great anti-ageing mechanism or it could be an early sign of cancer in them. Alternately, it may not mean both and could simply not be of any significant consequence.
The activation of telomerase in stem cells is an interesting field that needs further probing in order to understand the mechanism of ageing and to look into the possibilities of rejuvenation. One thing is very clear and that the process of ageing is a lot more complicated than is perceived and involves much more than the length of telomeres!