- Smith AJ et al. Chimeric antigen receptor (CAR) T cell therapy for malignant cancers: Summary and perspective. J Cell Immuntherap. 2016;2(2):59-68.
- Wang Z et al. New development in CAR T-cell therapy. J Hematol Oncol. 2017;10:53.
- CAR T-cell therapy impresses in multiple myeloma. Cancer Discov. 2017. doi: 10.1158/2159-8290.CD-NB2017-181.
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. The components of the immune system.
- CAR T-cell therapies - (https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/car-t-cell1.html)
- NCI Dictionary of Cancer Terms - (https://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=771302)
- CAR T cells: Engineering patients' immune cells to treat their cancers - (https://www.cancer.gov/about-cancer/treatment/research/car-t-cells)
- FDA approves CAR T-cell therapy to treat adults with certain types of large B-cell lymphoma - (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm581216.htm)
- Approval of gene therapies for two blood cancers led to an 'explosion of interest' in 2017 - (https://www.sciencenews.org/article/car-t-cell-gene-therapy-top-science-stories-2017-yir)
- Immunotherapy to treat cancer - (https://www.cancer.gov/about-cancer/treatment/types/immunotherapy)
- Hutch News: New insights into CAR T-cell therapy's potential side effects - (https://www.fredhutch.org/en/news/center-news/2017/10/car-t-cell-side-effects-study.html)
- NCI Dictionary of Cancer Terms - (https://www.cancer.gov/publications/dictionaries/cancer-terms)
What is CAR T- Cell therapy?
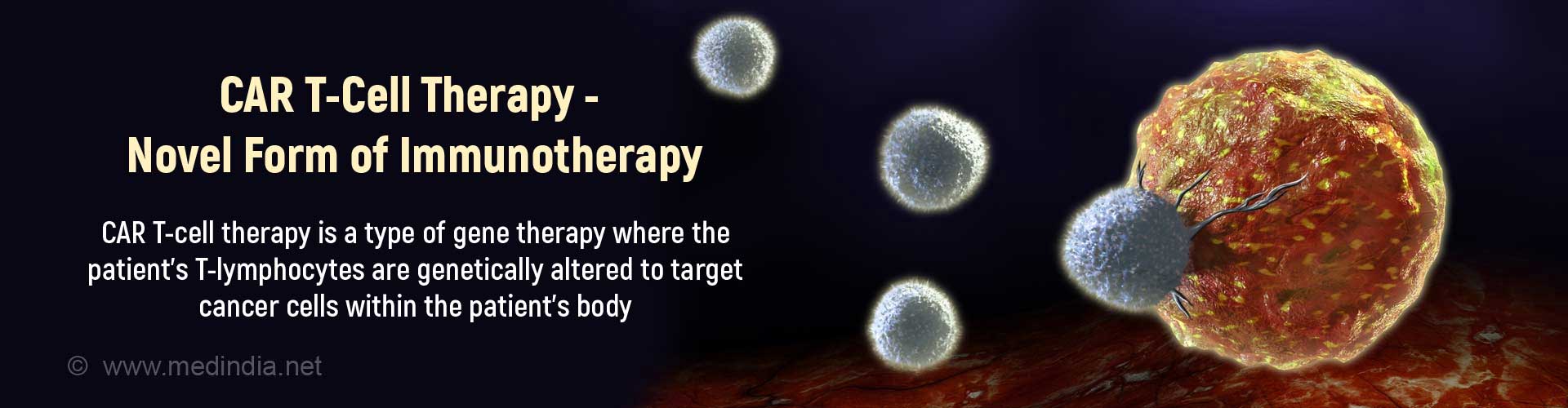
CAR T-cell (Chimeric Antigen Receptor T-cell) therapy involves using genetically altered patient’s T-lymphocytes to target the tumor cells of the patient. The FDA has approved 2 CAR T-cell therapies.
CAR T-cell therapy is a type of immunotherapy where the T-cells (a type of white cells) of the immune system (body defence system against disease) are genetically modified in the laboratory. These engineered T-cells are capable of expressing certain receptors on their surface by means of which they are able to recognize specific proteins (antigens or targets) on tumor cells in order to destroy them.
CAR T-cell therapy is a form of adoptive cell transfer treatment since it enhances the activity of T-cells to target and attack cancer cells.
Various Other Forms of Immunotherapy
Essentially, cancer treatment has been categorized into radiation, surgery, chemotherapy, and targeted therapy. Immunotherapy is now being hailed as the fifth pillar of treatment in cancer because it is now approved for clinical use.
Immunotherapy is basically a form of treatment that uses the body’s immune system (defence system) to fight diseases. The immune system comprises white blood cells, T-cells, and B-cells. Immunotherapy is not as frequently used as the other forms of cancer treatments.
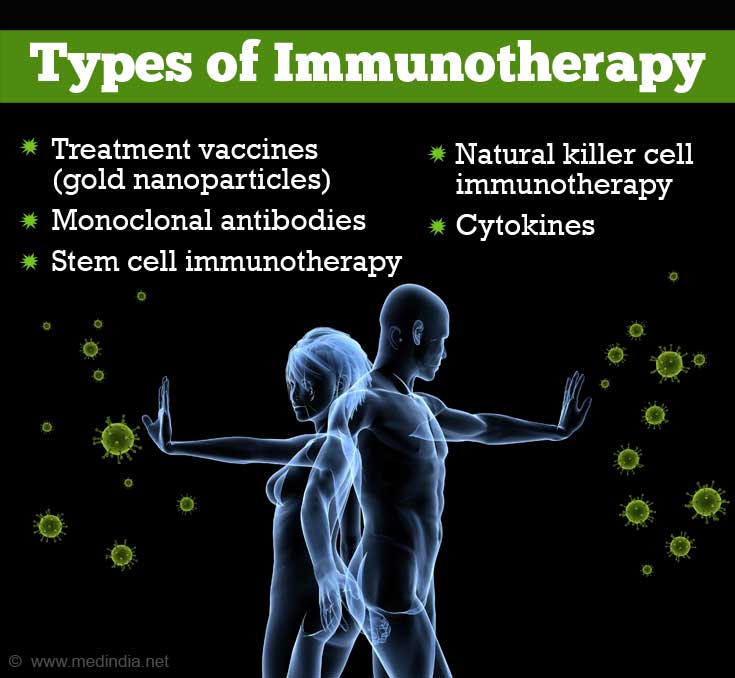
There are different types of immunotherapy. A few examples are:
- Treatment vaccines (gold nanoparticles)
- Tumor infiltrating lymphocytic immunotherapy
- Monoclonal antibodies
- Stem cell immunotherapy
- Natural killer cell immunotherapy
- Adoptive cell transfer
- Cytokines
What are the Recent Developments in CAR T-Cell Therapy?
2017 has been a breakthrough year for immunotherapy because the US FDA (Food and Drug Administration) approved the use of 2 CAR T-cell therapies for blood cancer treatment.
- In August, the FDA approved the use of Kymriah for treating B-cell acute lymphoblastic leukemia (ALL) in young adults and children.
- Similarly, Yescarta was FDA-approved in October to treat a type of non-Hodgkin lymphoma (large B-cell lymphoma) another form of blood cancer in adults.
However, CAR T-cell therapy is currently recommended only as a last resort for those patients who are resistant or refractory to any treatment for B-cell lymphoma and ALL.
What happens during CAR T-Cell Therapy?
CAR T-cell therapy involves removing, genetically altering, and transplanting T-cells back into the patient. Cytokine release syndrome is a main side effect.
Step 1 – T-cells are removed from the patient’s blood in a process called leukapheresis. The patient is required to sit still or lie in bed for 2 to 3 hours with 2 IV (intravenous) lines. One IV line draws the blood out while the second returns the blood into the body. The patient experiences tingling, numbness, and muscle spasms. Calcium levels drop and can be rectified with another IV line or by orally administering calcium.
Step 2 – The T-cells are separated from the white blood cells of the patient and genetically modified with a lentivirus or retrovirus in the laboratory making the T-cells express a chimeric antigen receptor (CAR) resulting in CAR T-cells.
The CAR targets an antigen that is found in tumor cells. A large number of CAR T-cells are required to attack the cancer cells. Hence, it may take 7 days to a few weeks to modify the T-cells.
Step 3 – Before the patient is treated with specific CAR T-cells, mild chemotherapy is administered to diminish the number of immune cells so CAR T-cells can attach to the specific cancer cells. Once binding occurs, CAR T-cells are able to increase in number and attack more cancer cells.
How does CAR T-Cell Therapy Work on Blood Cancer?
Currently CAR T-cells recognize and target CD19 protein on tumor B-cells in ALL and chronic lymphocytic leukemia (CLL).
- The CAR activates T-cells of the immune system in the same way that a foreign antigen activates the immune system with activation and release of cytokines which will result in tumor cell death and regression of cancer.
Evolution of CAR T-Cell Therapy Over Time
Since the development of CAR-T cell therapy in 1989, it has evolved with incorporation of better features to improve the efficacy. There are 4 generations of CAR T-cell therapy.
- The first generation contains the basic CAR structure without any costimulatory molecules. To be effective, it required concurrent administration of cytokines
- The second generation contains one costimulatory molecule along with the basic CAR structure and improves the stimulation and proliferation of T-cells.
- The third generation contains 2 costimulatory molecules with the basic CAR structure.
- The fourth generation named T cell redirected for universal cytokine-mediated killing (TRUCK) is used for targeting cytokines
The third and fourth generation treatments require careful analysis of safety profile and careful selection of costimulatory molecules.
What are the Possible Side Effects of CAR T-Cell Therapy?
As with any therapy, CAR T-cell therapy has its share of side effects and toxicities.
- Cytokine release syndrome (CRS) is one of the major side effects of CAR T-cell therapy. The CRS is associated with hypoxia (low oxygen levels), hypotension, and high fever. Corticosteroids treat CRS by decreasing the number of CAR T-cells and the number of cytokines produced. Similarly, Tocilizumab reduces the number of cytokines produced in CAR T-cell therapy.
- Neurotoxicity (headache, delirium, decreased consciousness, difficulty with speech) is another predominant side effect of CAR T-cell therapy. The condition is reversible and does not last too long. Cerebral edema or swelling in the brain is a rare complication that has resulted in a pharmaceutical company discontinuing its clinical trial due to patient mortality.

- Unnecessary targeting of other tissues by the CAR T-cell receptor results in autoimmune conditions. A favourable strategy is to treat patients with unmodified T-regulatory cells. Simultaneous treatment with modified CAR T-cells and unmodified T-regulatory (Treg) cells prevents inflammatory and autoimmune conditions. Sometimes with CAR T-cell therapy, a large number of normal B-cells are killed. This is known as B-cell aplasia. Hence, during treatment, immunoglobulins are administered so they have enough antibodies to resist infection.
- A weak immune system, reduced blood cell counts and major infections are other side effects.
- In rare instances, CAR T-cell therapy results in death. Researchers at the Fred Hutchinson Institute, Seattle, USA have developed an algorithm that identifies those at risk of side effects and even death from CAR T-cell therapy.
One method of identifying patients with fatal neurotoxicity based on their research is by detecting elevated levels of cytokines (MCP-1 and IL-6) and high fever (102ºC).