- Dengue vaccine research - (http://www.who.int/immunization/research/development/dengue_vaccines/en/)
- Dengue Vaccine Initiative - (http://www.denguevaccine.org/)
- Scott LJ. Tetravalent dengue vaccine: a review in the prevention of dengue disease. Drugs 2016 Sep; 76(13):1301-12. DOI - (10.1007/s40265-016-0626-8.)
- Ghosh A, Dar L. Dengue vaccines: challenges, development, current status and prospects. Indian J Med Microbiol 2015; 33(1): 3-15. PMID: 25559995. - (10.1007/s40265-016-0626-8.)
Why is a Dengue Vaccine Needed?
The burden of dengue in India is huge and the cost of treatment for complications like dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) is astronomical and can run into thousands of rupees, especially in corporate hospitals, where the expenses have to be borne out-of-pocket in the absence of health insurance.
The situation is aggravated by the fact that most government hospitals, especially in rural areas, lack the infrastructure and are ill-equipped to tackle complications of dengue infection. Therefore, the availability of a dengue vaccine in the public sector will bring about a paradigm shift from treatment to prevention. This is likely to dramatically bring down the number of dengue cases as well as substantially reduce the mortality and morbidity.
Impediments in Developing a Dengue Vaccine
Several dengue vaccine candidates have been under development over the past few decades, but none have been successful, until now. The difficulty in developing a dengue vaccine stems from the fact that there are four kinds (serotypes) of dengue virus (DENV) that circulate, of which one type predominates in a particular dengue season.
Weakening the virus (technically termed as attenuation) by chemical treatments so that it does not cause disease, yet is still strong enough to stimulate the immune system (defense system of the body) to generate a protective immune response is very tricky, especially since there are four serotypes that require equal levels of attenuation. This problem has been solved in the new dengue vaccine developed by Sanofi Pasteur.
Dengvaxia: The Sanofi Pasteur Dengue Vaccine
An internationally available dengue vaccine called Dengvaxia (CYD-TVD), developed by Sanofi Pasteur, Lyon, France has been extensively evaluated in more than 40,000 individuals in 15 different countries, including India.
The Sanofi vaccine is recommended by the World Health Organization (WHO) for use in individuals aged between 9 and 45 years in areas of the world where dengue is prevalent. Dengvaxia is a live-attenuated tetravalent (containing all four DENV serotypes – DENV1, DENV2, DENV3 and DENV4) dengue vaccine produced by recombinant DNA technology or genetic engineering.
The vaccine has been evaluated in a 3-dose schedule (0/6/12 months) in Phase III clinical trials and was first registered in Mexico in December 2015 and first commercially available in 2016 in the Philippines and Indonesia. The vaccine has since been adopted in 7 other dengue-endemic countries around the globe, namely, Brazil, Paraguay, El Salvador, Costa Rica, Peru, Guatemala, and Singapore, where more than 500,000 people have already been vaccinated with over 1.5 million doses distributed so far.
The Government of India is exercising caution on the introduction of the Sanofi dengue vaccine, as the company is seeking exemption from carrying out Phase III clinical trials in India, arguing that it has already been extensively tested and that the exemption would fast-track the introduction of the vaccine. Early introduction of the vaccine will help in tackling the disease in India, where dengue is a huge public health problem.

Other Dengue Vaccine Candidates
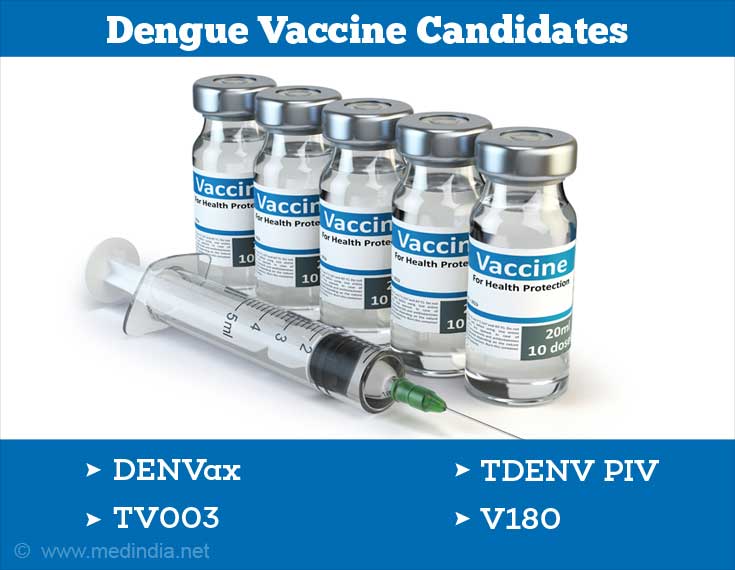
Several other dengue vaccine candidates are under different stages of development in various countries. A few important ones are briefly highlighted below:
- DENVax: This vaccine, developed by Takeda, consists of a chimera (mosaic) of the four dengue serotypes. This dengue vaccine candidate is currently undergoing Phase III clinical trials.
- TV003: This vaccine has been developed by the National Institute of Allergy and Infectious Diseases (NIAID), USA. TV003 is a live-attenuated vaccine that contains a mixture of the four dengue serotypes as separate vaccine formulations. The vaccine is currently undergoing Phase II clinical trials.
- TDENV PIV: This is an inactivated (killed) tetravalent (containing DENV1-4) vaccine formulation developed jointly by GlaxoSmithKline and the Walter Reed Army Institute of Research, USA. It is currently undergoing Phase I/II clinical trials.
- V180: This is a subunit (fragment of a protein antigen) protein vaccine produced in the cells of fruit fly (Drosophila). This vaccine, developed by Merck, has completed Phase I clinical trials as of December 2014.
Indian Efforts
The International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi has developed a dengue vaccine candidate, which has been extensively evaluated in various animal models, including monkeys. This vaccine candidate is currently awaiting clinical trials in humans. The scientists are hopeful that a “Made-in-India” dengue vaccine could be available within a span of 4-5 years from now.
What is Dengue?
Dengue is a viral disease caused by the dengue virus, which is transmitted by the bite of Aedes aegypti mosquitoes. The disease has a global distribution in all tropical and sub-tropical regions where the Aedes mosquito is present. The virus has an affinity for the blood and its components, which results in disease manifestation.
Dengue Burden in India
The disease burden is staggering as revealed by a 2014 study conducted by the National Institute of Health and Family Welfare (NIHFW), New Delhi. The study found that the annual average clinically diagnosed dengue cases in India was almost 6 million, or 282 times the annual reported number during 2006-2012.
Dengue Types & Symptoms
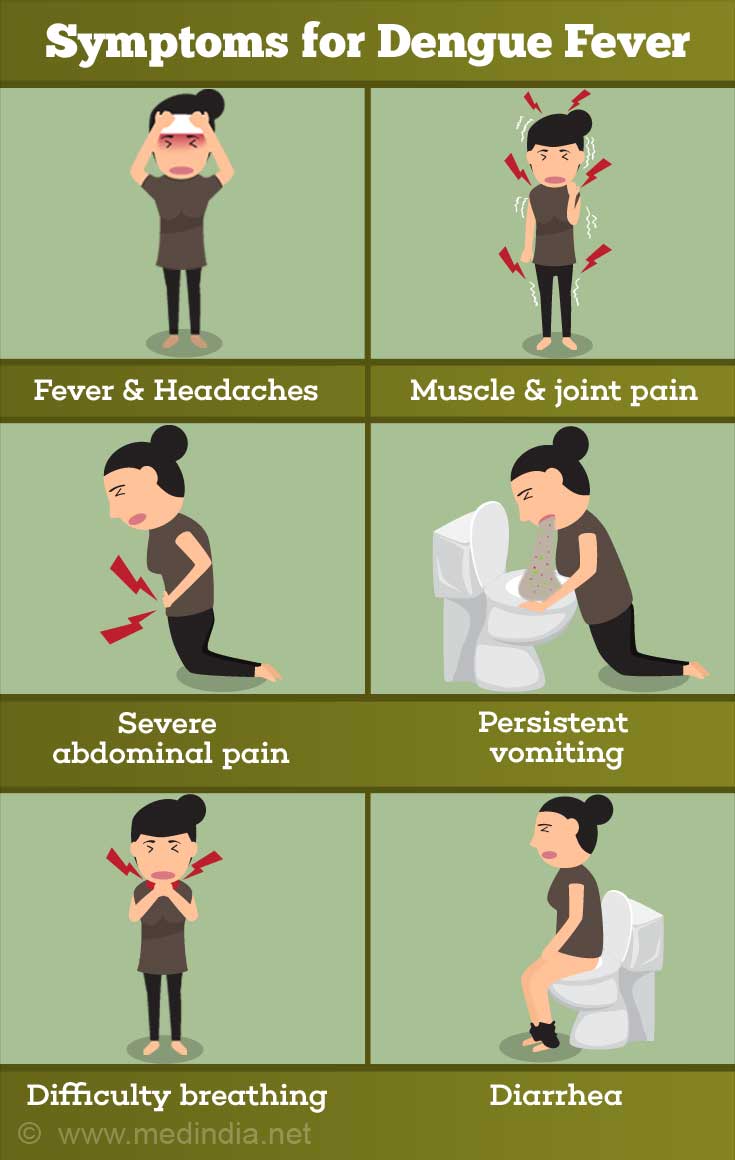
Most common - The most common form of the disease is dengue fever, which is characterized by severe bone and joint pain (for which it is also called “breakbone fever”), muscle pain, headache, fever, swollen lymph nodes, rash, and generalized fatigue and exhaustion.
Dengue hemorrhagic fever (DHF) - Presence of fever, rash and headache are the three characteristic symptoms (“dengue triad”) of dengue. Dengue hemorrhagic fever (DHF) is a more severe form of the disease, largely affecting children below the age of 10 years. As the name suggests, the condition is characterized by widespread hemorrhage (bleeding), abdominal pain, in addition to fever, rash and headache. A potentially fatal complication is dengue shock syndrome (DSS), which is characterized by circulatory collapse and death, if not treated promptly.
Treatment & Prevention
There is no specific treatment for dengue and NO antivirals against dengue are currently available. Dengue must be treated in a hospital setting and it primarily involves supportive therapy. Currently, the mainstay of prevention is mosquito vector control.
Health Tips
- Avoid mosquito bites: While in dengue endemic regions, cover all exposed parts of the body to minimize mosquito bites. Wear full-sleeved shirts, full-trousers, and hats.
- Use mosquito repellent creams: You can apply commercially available mosquito repellent creams on the hands and feet, but avoid applying on the face.
- Remember that Aedes are daytime-biters: Since Aedes mosquitoes bite during the daytime, you should be careful about unnecessary bites during the day. Use a mosquito net for maximal protection while indoors.
- Use indoor residual spraying (IRS) on walls: Aedes mosquitoes generally sit on the walls inside the house. Using IRS can effectively kill Aedes mosquitoes sitting on the walls.
- Seek medical help if you have symptoms of dengue infection: Remember that early diagnosis and prompt treatment are very important for preventing complications of dengue infection. Therefore, consult a doctor immediately if you have symptoms of dengue.