- Hib Vaccine - (https://www.cdc.gov/vaccines/hcp/vis/vis-statements/hib.pdf)
- Kelly DF, Moxon ER, Pollard AJ. Haemophilus influenzae type b conjugate vaccines. Immunology. 2004;113(2):163-174 - (doi:10.1111/j.1365-2567.2004.01971.x.)
- Haemophilus influenzae type b conjugate vaccines - (http://www.who.int/biologicals/areas/vaccines/haemophilus/en/)
- Haemophilus influenzae type b (Hib) - (http://www.who.int/biologicals/areas/vaccines/haemophilus/haemophilus_influenzae_typeb_Hib/en/)
- Haemophilus b Conjugate Vaccines for Prevention of Haemophilus influenzae Type b Disease Among Infants and Children Two Months of Age and Older Recommendations of the ACIP - (https://www.cdc.gov/mmwr/preview/mmwrhtml/00041736.htm)
What is Hib Vaccine?
The Hib vaccine protects against severe bacterial infections like meningitis, pneumonia, and epiglottitis caused by the bacterium H. influenzae type b. The Hib bacteria spread through sneezing, coughing, and nasal secretions from an infected person to another person.
Brief Overview Of Haemophilus influenzae Type B (Hib) Disease
The Hib disease is caused by a gram-negative bacterium named Haemophilus influenzae that commonly infects the upper respiratory tracts of children. The germs spread among children through the transfer of nasal secretions during sneezing and coughing. Although children under 5 years are most susceptible to Hib infections, immune-compromised adults can also get infected.
The Hib disease can become invasive when the germs enter the lungs or the bloodstream. Only a minority of those exposed or who are carriers of the organism suffer from the invasive disease. The H influenzae strains that have a polysaccharide (sugar) capsule or coat cause a more serious disease. The H influenzae, type b (Hib) (one of the 6 capsular types) is responsible for more than 90% of the invasive or systemic infections.
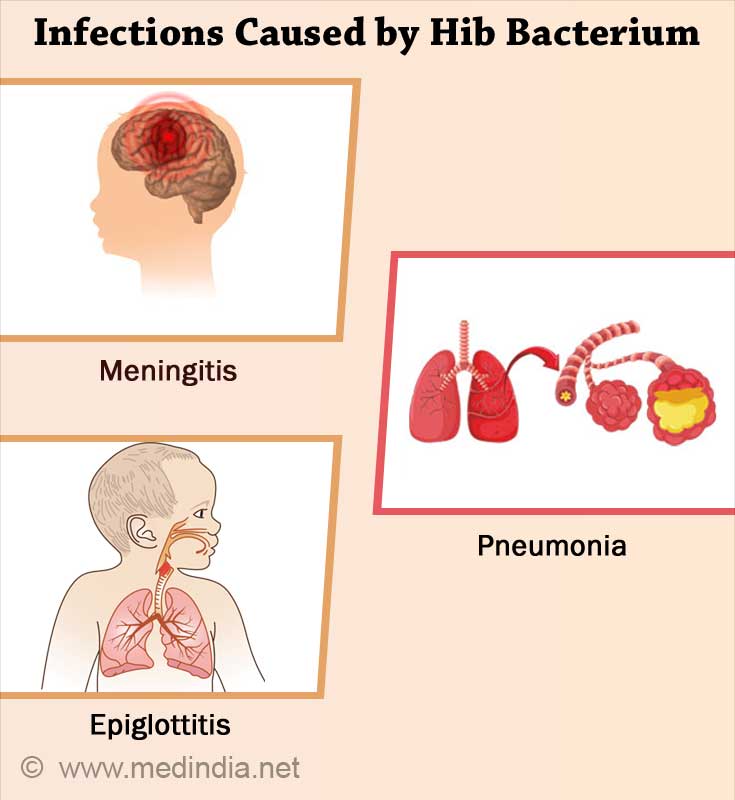
Infections that can be caused by the Hib bacterium are:
- Meningitis (an infection of the tissue lining the brain and spinal cord that can lead to brain damage and deafness)
- Pneumonia (lung infection)
- Epiglottitis (a severe throat infection that causes swelling of the throat making it hard to breathe)
- Infections of the blood, joints, bones, and covering of the heart (pericardium)
Hib disease is a significant public health concern in many parts of the world; the H. influenzae type b bacterium can cause a potent form of the disease in around 3 million people every year. Another rising fear is the increasing resistance of the Hib bacteria to antibiotic agents that have been reported from many parts of the world. This can be prevented if the Hib vaccine is used. Thus far, the Hib vaccine has been a big boon to society by cutting down the number of invasive Hib disease cases by more than 99%.
How does the Hib Vaccine Work?
The Hib vaccine is an inactivated vaccine. It is made chemically by bonding the polysaccharide called polyribisylribitol phosphate or PRP (that makes up the surface capsule of the bacterium) to a carrier protein. This process is known as conjugation, and hence the Hib vaccine is a polysaccharide conjugate vaccine.
Hib conjugate vaccines induce the production of protective circulating antibodies against PRP (anti-PRP) which protects the individuals from attack by the H. influenzae type b bacteria. The vaccine also decreases the nasopharyngeal colonization of Hib, thereby reducing the chances of the spread of the infection.
The conjugate vaccine produces a better immune response as it has the advantage of being recognized by both the B cells and the T cells (B lymphocytes and T lymphocytes are white blood cells involved in immunity). The polysaccharide vaccine (that was used earlier and did not have the carrier protein) was only recognized by the B cells (this kind of interaction is called t-independent or TI), due to which the immunity may not have lasted for a long time.
What are the Types of Hib Vaccines?
Liquid or lyophilized preparations of the purified or synthetic sugar, PRP is conjugated either to the diphtheria toxoid (PRP-D), a diphtheria toxoid-like protein (PRP-HbOC), tetanus toxoid (PRP-T), or meningococcal outer membrane protein (PRP-OMP). These vaccines are called monovalent vaccines.
The conjugate vaccines differ from each other depending which protein carrier is used, the size of the polysaccharide, the method of chemical conjugation, and also the use of a spacer or a linking moiety between the PRP and protein carrier.
The Hib vaccine can also be given as part of a combination vaccine, where two or more types of vaccines are combined and administered as a single shot to protect against more than one disease. Hib combination vaccines contain diphtheria, tetanus, and pertussis (DPT-aP) vaccine, sometimes along with hepatitis B and/or the inactivated poliovirus vaccine, IPV.
Commercially, there are three types of monovalent vaccines and one combination vaccine licensed for use by the Food and Drug Administration (FDA).
Who should get the Hib Vaccine?
- All infants should receive Hib vaccine as part of their routine immunization. As Hib disease is rare in children older than five years, it is not routinely recommended for people five years or older.
- High-risk individuals include those with sickle cell disease (which damages the spleen), immunosuppressed from cancer chemotherapy, people 5 to 18 years old with HIV infection, and those who have undergone splenectomy (removal of the spleen), or a bone marrow transplant. These individuals have reduced immunity, which puts them at risk for Hib infection.

What is the Dosage Schedule for the Hib Vaccine?
The first primary dose can be given as early as 6 weeks. Any of the monovalent vaccines can also be used for older children and adults that need Hib vaccination.
The number of doses (either 2 or 3 primary doses) of the vaccine depend on the type of vaccine used
The recommended ages for the doses are:
- First Dose: 2 months of age
- Second Dose: 4 months of age
- Third Dose: 6 months of age (if needed, depending on the brand of the vaccine)
- Final/Booster Dose: 12-15 months of age
Children between 6 weeks and 2 years of age who have suffered from a Hib infection should also be administered the vaccine after recovery.
How is Hib Vaccine Given?
The Hib vaccine is given as an injection into the muscle.
What are the Contraindications for Hib vaccination?
Hib vaccine should not be given to the following individuals:
Those who had a severe allergic reaction to a previous dose of Hib vaccine or any part of the vaccine
Children less than six weeks of age
Persons who have a moderate or severe acute illness, who should receive the vaccine only after recovery from the illness.
What are the Side Effects of the Hib Vaccine?
Usually, there are no side effects associated with vaccination. Minor side effects include warmth, soreness, and redness at the injection site and mild fever. Serious problems are mainly allergic reactions to vaccine components.
If there are signs of a severe allergic reaction (hives, swelling of the face and throat), difficulty breathing, fast heartbeat, dizziness, and weakness), very high fever, or unusual behavior, please consult the doctor immediately.