A clinical trial of this system shows a significant reduction in blood pressure in patients who suffer from severe hypertension and cannot control their condition with medications or lifestyle changes
Patients suffering from severe hypertension and those who cannot control their conditions with medications need not worry now --- a new device system is being introduced for their rescue.
The new device known as Rheos System was first implanted in U.S at the University of Rochester Medical Center as part of a clinical trial. The early findings of Rheos system showed a significant reduction in the blood pressure in patients suffering from severe hypertension. University of Rochester Medical Center cardiologist John Bisognano, M.D., Ph.D., and Minneapolis-based device-maker CVRx at the American Heart Association 2006 Scientific Sessions in Chicago shared early findings this week.The ongoing study is assessing the safety and clinical efficacy of the RheosTM Baroreflex Hypertension TherapyTM System, an implantable device for the treatment of hypertension in patients with drug-resistant hypertension, who have a systolic blood pressure of 160 mmHg or greater. The University of Rochester implanted the first device in the U.S. in March 2005, and performed a total of three of the initial 10 implantations.
"The Rheos System is a novel device that activates the carotid baroreflex, the body's own system for regulating blood pressure," Bisognano said. "We are pleased with the clinical results to date and look forward to expanding the clinical evaluation of the Rheos System. New approaches to the widespread, chronic and costly problem of hypertension are needed. The Rheos System has the potential to prevent the progression to more serious illnesses, including heart and kidney disease, stroke and death."
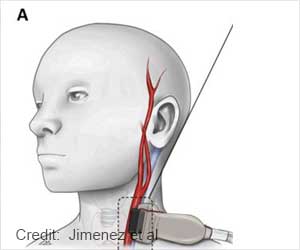
The system works by electrically activating the baroreflex system based in the carotid arteries in the neck. Low-level electrical stimulation to this area sends signals to the brain, "telling" it to take action to lower blood pressure through a variety of mechanisms, including blood vessel dilatation, heart rate reduction and promotion of fluid excretion by the kidneys.
In this way, the system provides a physiologic approach to reducing high blood pressure by allowing the brain to direct the body's own control mechanisms. It consists of a battery-powered implantable generator, which is inserted under the skin near the collarbone, and two carotid sinus leads, which run from the generator to the left and right carotid sinus in the neck. While implantation is slightly more involved, the general principle is similar to the implantation of cardiac pacemakers.
The trial is designed to assess device safety and efficacy in patients with systolic blood pressure of 160 mmHg or greater, despite being on at least three anti-hypertension medications, including one diuretic.
Advertisement
In October, CVRx received a conditional investigational device exemption (IDE) approval from the U.S. Food and Drug Administration to begin a pivotal clinical trial that will evaluate the safety and effectiveness of the RheosTM Baroreflex Hypertension TherapyTM System in a much larger number of patients. The University of Rochester team recently implanted a fourth device as part of the study.
Advertisement
Source-Eurekalert
SRI