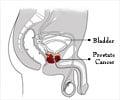
Abiraterone, the new prostate cancer drug discovered by British researchers, is showing considerable promise. The pill could be available in the market in three years, they hope.
Abiraterone, the new prostate cancer drug discovered by British researchers, is showing considerable promise. The pill could be available in the market in three years, they hope.
Scientists at the Royal Marsden Hospital in South-West London are bubbling with confidence that their discovery could turn out to be a wonder drug.Tumours shrank in up to 80 per cent of patients treated. There were also dramatic" falls in levels of prostate-specific antigens – which indicate the severity of prostate cancer. Similar results have been observed in second-phase trials involving about 250 men, details of which have yet to be published.
Dr Johann de Bono, the lead researcher on the study, said: "Patients in this study have been monitored for up to two-and-a-half years and, with continued use of abiraterone, were able to control their disease with few side effects.”
They had very aggressive prostate cancer, which is exceptionally difficult to treat and almost always fatal.
And they were able to do so with just four pills a day. The drug works even if the cancer has spread beyond the prostate, such as to the bone.
“We hope that abiraterone will eventually offer them real hope of an effective way of managing their condition and prolonging their lives. My vision is to make chemotherapy obsolete,” Bono said.
Advertisement
However, this treatment does not block the production of male hormones elsewhere in the body. Abiraterone inhibits an enzyme called CYP450c17, which is critical to the production of the male hormones — not only in the testes, but also at other sources.
At the European Society for Medical Oncology Conference Lugano 2007, it was reported that he new drug reduced prostate-specific antigen (PSA) levels and also resulted in tumor shrinkage. Decline in PSA levels was accompanied by evidence of tumor shrinkage on scans, drops in circulating tumor-cell counts, and improvements in symptoms, they noted.
Advertisement
Many patients also have radiotherapy, to reduce associated pain in the bones. This can be dangerous too, damaging organs and leaving patients exhausted and with little quality of life. Abiraterone seems to offer a way out.
John Neate, chief executive of the Prostate Cancer Charity, said: 'This is an exciting development which has been eagerly anticipated. Advanced prostate cancer is very difficult to treat as, after time, it stops responding to conventional ways of controlling the male hormone.
'We look forward to the results of the larger trials already under way or being planned for this drug to prove its potential effectiveness.'
As the drug is newly in development, it is not yet known how many years' extra survival it can bring. But patients on the trial have so far lived longer than the estimated 12 or 18 months.
Abiraterone is now being used in a 1,200-patient international study, including at ten sites across the UK. If it is licensed as expected in 2011, it will have to await approval by the rationing watchdog NICE before it is made freely available across the NHS.
Drugs usually go through three phases of clinical trials before pharmaceutical companies apply for a licence to make them more widely available. This is to prove their safety and effectiveness in ever-larger groups of patients.
Small trials alone may not be enough to show up side-effects that emerge during much larger studies.
Dr de Bono describes prostate cancer as the 'Cinderella cancer' because it receives just a quarter of the funding of breast cancer - £10million against £40million last year - even though it kills just as many people.
Source-Medindia
GPL/M


![Prostate Specific Antigen [PSA] Prostate Specific Antigen [PSA]](https://www.medindia.net/images/common/patientinfo/120_100/prostate-specific-antigen.jpg)