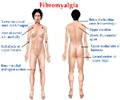
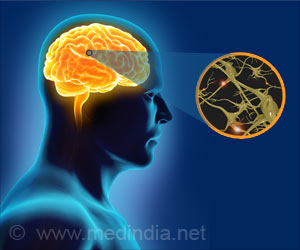
Abnormal pain signal processing could be the reason why fibromyalgia patients experience pain and are unresponsive to popular class of pain relievers known as opioids.
"Although we have known for some time that the brain is a key player in the pathology of fibromyalgia, we have yet to understand how pain regulation is disrupted in this condition," says Richard E. Harris, PhD, assistant professor at the University of Michigan, Ann Arbor, Mich., and lead investigator of the study.
Previous studies indicate that fibromyalgia patients have increased sensitivity to temperature, touch, and pressure. Moreover, some of Dr. Harris''s previous work demonstrated that people with fibromyalgia produce an increased amount of endogenous opioid peptides (also known as endorphins that naturally relieve pain) that act on the brain''s μ-opioid receptors to "naturally" reduce pain. Other work by this same group showed that the fibromyalgia brain displays an enhanced response to painful stimuli, suggesting a problem with pain processing. This current study sought to determine if these two factors, altered function of μ-opioid receptors and enhanced brain response to pain, actually occur simultaneously within the same group of people with fibromyalgia - and within the same brain regions.
To answer this question, researchers from the University of Michigan measured the change in blood flow in the brains of 18 patients with fibromyalgia following a painful stimulus, using functional magnetic resonance imaging. They also measured the μ-opioid receptor binding availability with additional tests. These data were collected before and after acupuncture and sham acupuncture (which is essentially placebo acupuncture) treatment designed to reduce pain. The association between the brain''s response to pain and the binding of μ-opioid receptors was then examined.
The study revealed a strong negative association between the brain''s response to pain and the binding availability of μ-opioid receptors: the lower the receptor binding availability the greater the brain''s response to pain. A positive correlation was also observed in a classic pain prevention region, the right dorsolateral prefrontal cortex. Importantly these associations were also related to the pain sensations patients reported.
For the first time, this study shows that μ-opioid receptor binding is tightly associated with the brain''s response to pain in fibromyalgia. The data leads researchers to speculate that some individuals with fibromyalgia may have a down-regulation or decrease in opioid receptor activity that may exaggerate pain sensitivity. Moreover, these same individuals are likely to not benefit from opioid medications as they may have fewer functioning receptors.
Advertisement
Funding for this study was provided by the National Institute of Health and the Department of Defense.
Advertisement
Learn more about living well with rheumatic disease as well as rheumatologists and the role they play in health care. Also, discover the ACR''s Simple Tasks campaign, which highlights the severity of rheumatic diseases and the importance of early and appropriate referral to a rheumatologist.
Editor''s Notes: Richard E. Harris, PhD, will present this research during the ACR Annual Meeting at the Walter E. Washington Convention Center at 11:15 AM on Tuesday, November 13 in Hall E. Dr. Harris will be available for media questions and briefing at 8:30 AM on Monday, November 12 in the on-site press conference room, Room 203 A-B.
Presentation Number: 2450
Evoked Pain Brain Response Is Associated with Reduced μ-Opioid Receptor Binding in Fibromyalgia
Richard E. Harris: University of Michigan
Richard E. Harris (University of Michigan, Ann Arbor, Mich.)
Heng Wang, (University of Michigan, Ann Arbor, Mich.)
Daniel J. Clauw (University of Michigan, Ann Arbor, Mich.)
Jon-Kar Zubieta (University of Michigan, Ann Arbor, Mich.)
Background/Purpose: Previous studies indicate that fibromyalgia (FM) patients have augmented clinical and brain responses to painful stimuli (i.e. hyperalgesia/allodynia), as well as increased production of endogenous opioids, and reduced μ-opioid receptor (MOR) binding. However, it is not known if these factors co-occur within the same individual or if these factors act independently. We performed a longitudinal investigation using functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) in chronic pain patients diagnosed with FM to address this question. If these factors operate in the same individual, we expected an inverse correlation between changes in fMRI evoked pain activity and MOR binding potential (BP).
Methods: fMRI and PET imaging sessions were performed on 18 female opioid-naïve FM patients (age 45.4+/- 13.0). Each participant underwent 4 weeks of non-pharmacological treatment. Before and after treatment, each patient underwent an fMRI scan with varying levels of pressure pain applied to the thumb as well as a 90-minute [11C]carfentanil PET scan under resting conditions. After quantification of the PET data with Logan plots, fMRI images and preprocessing of PET data were performed with statistical parametric mapping (SPM5). fMRI and PET scans were normalized to the same template. Difference images before and after treatment were calculated for both the fMRI contrast and PET images. A whole-brain voxel-by-voxel correlation analysis between the fMRI and PET difference images were carried out using the Biological Parametric Mapping toolbox. Activation clusters were defined based on a correlation coefficient, with |R|>=0.6 uncorrected. Clinical pain was assessed with Short Form McGill Pain Questionnaire (SFMPQ).
Results: Negative correlations between the change in the fMRI blood oxygenation level dependent (BOLD) signal and MOR BP were observed in multiple regions involved in pain processing and modulation: right posterior insula R=-0.82,P=0.0004; left medial insula R=-0.82, P=0.0003; left orbital frontal cortex R=-0.75, P=0.0004; right amygdala R=-0.68, P=0.002; brainstem R=-0.71, P=0.0009. Positive correlations were observed in right DLPFC R=0.66, P=0.003; posterior cingulate R=0.62, P=0.006; right putamen R=0.72, P=0.0008. Changes in both functional imaging outcomes were negatively associated with changes in clinical pain: BOLD in right DLPFC and clinical pain SFMPQ; R=-0.52, P=0.03; MOR BP in left medial insula and SFMPQ present pain R=-0.51, P=0.03.
Conclusion: We find strong longitudinal associations between evoked pain activations suggestive of hyperalgesia, and µ-opioid receptor availability (binding potential, BP) within the same brain regions, in individual FM patients. Positive associations were also observed between BOLD responses, and μ-opioid receptor BP (in opposite directions) with respect to clinical pain. These data suggest that the µ-opioid system is somehow involved in the pathogenesis of FM, and may even help explain why these patients are generally not felt to respond to narcotic analgesics, and may even be made worse when these drugs are used therapeutically.
Disclosure:
Richard E. Harris: Pfizer Inc
Heng Wang: None, Daniel J. Clauw: Pfizer Inc, Forest Laboratories, Merck, Nuvo, Jon-Kar Zubieta, None
Source-Newswise