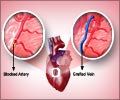
A new study has revealed that the risk of death was not lower among coronary artery bypass graft surgery patients who were given the agent acadesine
Mark F. Newman, M.D., of Duke University Medical Center, Durham, N.C., and colleagues conducted a study designed to more definitely test whether acadesine could reduce the composite of all-cause mortality, nonfatal stroke, or need for mechanical support for severe left ventricular dysfunction (SLVD) occurring during and after CABG surgery through postoperative day 28. The Reduction in Cardiovascular Events by Acadesine in Patients Undergoing CABG (RED-CABG) trial, a randomized, placebo-controlled study included intermediate- to high-risk patients (median [midpoint] age, 66 years) undergoing nonemergency, on-pump CABG surgery at 300 sites in 7 countries. The majority of participants were white men with a history of hyperlipidemia, diabetes, family history of cardiovascular disease, and previous percutaneous coronary intervention (procedures such as balloon angioplasty or stent placement used to open narrowed coronary arteries). Enrollment occurred from May 2009 to July 2010. Eligible participants were randomized to receive acadesine or placebo beginning just before anesthesia induction.
The trial was stopped after 3,080 of the originally projected 7,500 study participants were randomized because early analysis indicated a very low likelihood of a statistically significant efficacious outcome. The final sample size consisted of 2,986 patients in the intention to treat analysis. The researchers found that the primary efficacy outcome (composite of all-cause mortality, nonfatal stroke, or need for mechanical support for SLVD) was observed in 5 percent of participants overall. There was no significant difference between the placebo and acadesine groups, with incidence rates of 5.0 percent and 5.1 percent, respectively.
Additional exploratory efficacy end points, including length of mechanical ventilation, intensive care unit and hospital stay, and quality of life, did not reach statistical significance.
"These findings illustrate inherent risks of using promising meta-analysis results to plan ‘confirmatory'' clinical trials. There are several potential explanations for the negative results we observed. One consideration is the decision around study eligibility criteria and the overall primary end point of the trial," the authors write. They add that the lack of benefit of acadesine may be related to the dosing regimen used.
The researchers conclude that the incidence of the primary composite end point of 5 percent indicates the need for continued investigation into therapies to reduce perioperative morbidity and mortality. "However, effective therapies remain elusive."
(JAMA. 2012;308[2]:157-164. Available pre-embargo to the media at http://media.jamanetwork.com)
Advertisement
Advertisement