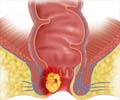
Advaxis announces the first stage of phase 2 clinical trial (dosing requirement) in fawcett study for axalimogene filolisbac (AXAL) used in anal cancer treatment

Phase 2 clinical studies mainly involve two stages, where the first stage concentrates on dosing requirements (fixing the dose) while the second stage looks after the efficacy of the drug at the prescribed dose. // The first stage of the Phase 2 clinical trial will be carried on 31 patients with recurrent anal cancer. Dose of 1x109 colony forming unit (CFU) will be given for every three weeks for a period of two years. The main aim of the study is to evaluate the safety and efficacy of AXAL in patients with HPV- associated metastatic cancer who have received prior treatment for the disease.//
‘Advaxis announces first stage of phase 2 clinical trial for axalimogene filolisbac (AXAL), a new treatment option for metastatic or recurrent anal cancer.
’





Daniel J. O’Connor who is the President and Chief Executive officer of Advaxis stated that the company has put forward an important step for the development of AXAL which is known as the Fawcett study (Fighting Anal Cancer with CTL Enhancing Tumor Therapy) as people with anal cancer need new options for treatment. The company mainly evaluates two areas which include monotherapy with AXAL and immune check-point inhibitor therapy with AXAL.//The Fawcett study was named after Farrah Fawcett, an American actress and artist who died due to HPV associated anal cancer. It is also noted that Advaxis company was honored with “Medical Visionary Angel Award” by the Fawcett Foundation in 2015 for its efforts to treat anal cancer.//
Source-Medindia