New AI-driven approach can perform similar to human experts by identifying diagnostic blast leukemia cells at least as good as a trained cytologist expert.
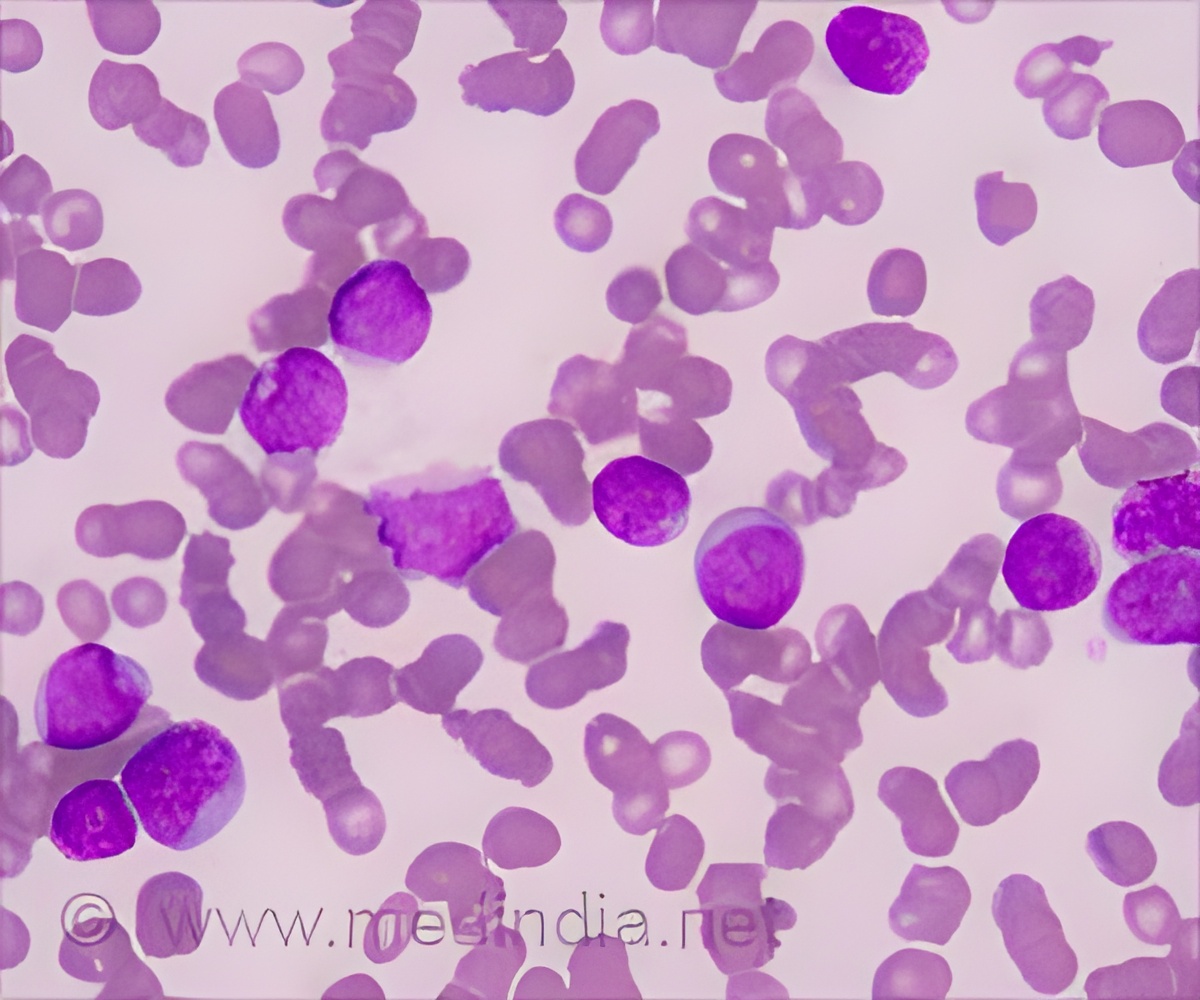
‘The AI-driven solution can identify diagnostic blast cells at least as good as a trained cytologist expert.
’





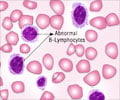
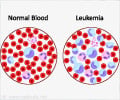
To improve evaluation efficiency, a team of researchers at Helmholtz Zentrum München and the University Hospital, LMU Munich, trained a deep neuronal network with almost 20.000 single cell images to classify them. The team lead Dr. Carsten Marr and medical doctoral student Dr. Christian Matek from the Institute of Computational Biology at Helmholtz Zentrum München as well as Prof. Dr. med Karsten Spiekermann and Simone Schwarz from the Department of Medicine III, University Hospital, LMU Munich, used images which were extracted from blood smears of 100 patients suffering from the aggressive blood disease AML and 100 controls. The new AI-driven approach was then evaluated by comparing its performance with the accuracy of human experts. The result showed that the AI-driven solution is able to identify diagnostic blast cells at least as good as a trained cytologist expert.Applied research through AI and Big Data
Deep learning algorithms for image processing require two things: first, an appropriate convolutional neural network architecture with hundreds of thousands of parameters; second, a sufficiently large amount of training data. So far, no large digitized dataset of blood smears has been available, although these samples are used pervasively in clinics. The research group at Helmholtz Zentrum München now provided the first large data set of that type. Currently, Marr and his team are collaborating closely with the Department of Medicine III at the University Hospital of LMU Munich and one of the largest European Leukemia laboratories, the Munich Leukemia Laboratory (MLL), to digitalize hundreds of patient blood smears more.
"To bring our approach to clinics, digitization of patients' blood samples has to become routine. Algorithms have to be trained with samples from different sources to cope with the inherent heterogeneity in sample preparation and staining," says Marr. "Together with our partners, we could prove that deep learning algorithms show a similar performance as human cytologists. In the next step, we will evaluate how well other disease characteristics, such as genetic mutations or translocations, can be predicted with this new AI-driven method."
This method showcases the applied power of AI for translational research. It is an extension of the pioneering work of Helmholtz Zentrum München on single-cell classification in blood stem cells (Buggenthin et al., Nature Methods, 2017), which has been awarded the Erwin Schroedinger Prize of the Helmholtz Association in 2018. The study was supported by the SFB 1243 of the German Research Foundation (DFG) and by a Ph.D. scholarship of the German José Carreras Leukaemia Foundation to Dr. Christian Matek.
Advertisement