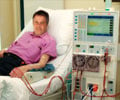
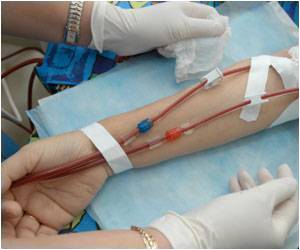
ALung obtained Expedited Access Pathway designation for its Hemolung RAS that removes carbon dioxide from blood, letting the lungs rest without having to undergo ventilation.
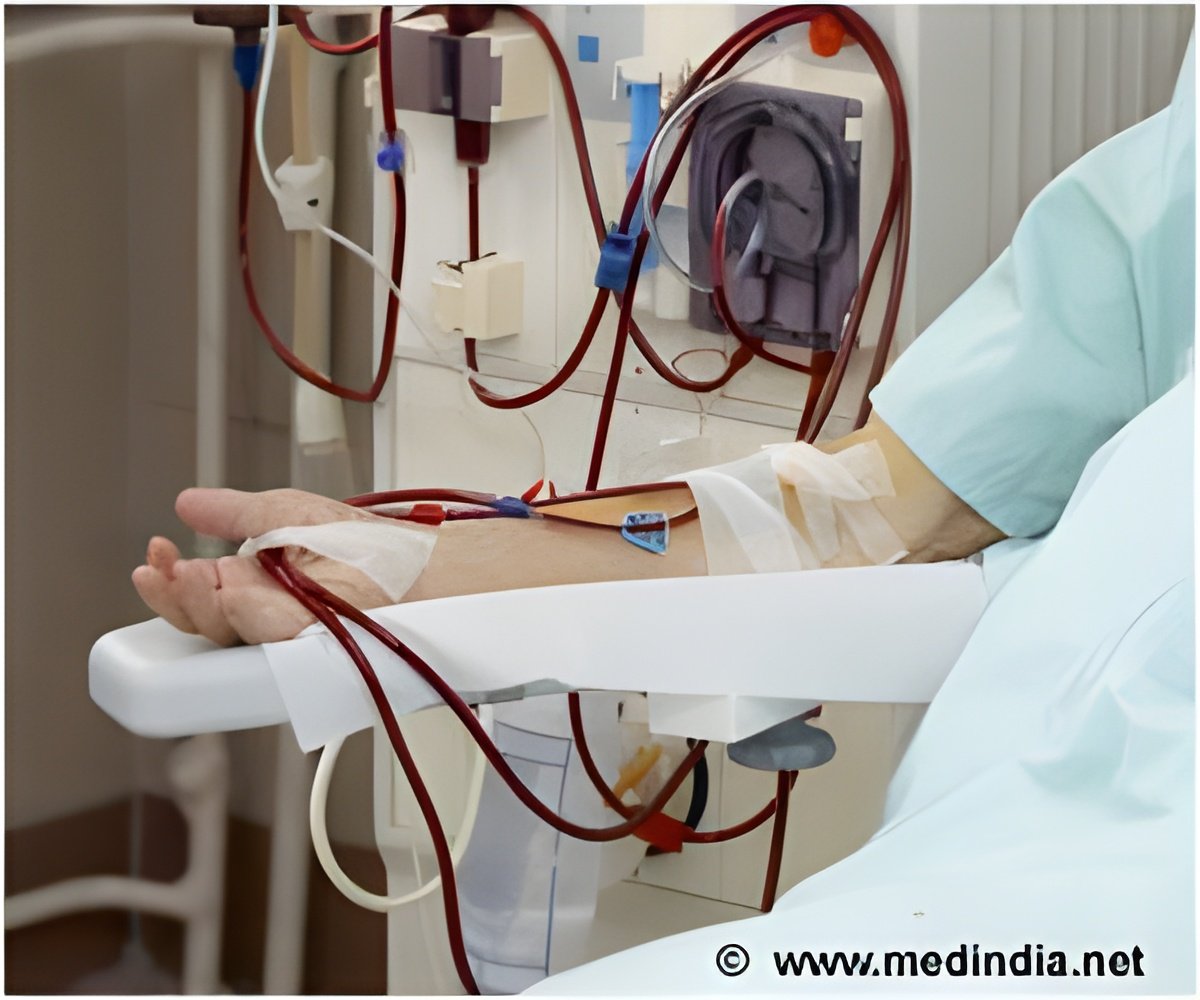
‘ALung obtained Food and Drug Administration’s Expedited Access Pathway designation for its Hemolung Respiratory Assist System that eliminates carbon dioxide in the blood, letting the lungs rest without having to undergo ventilation.’





Expedited Access Pathway will permit to introduce life saving devices quicker, as long as they meet certain unmet needs.ALung’s technology was considered as one of the most interesting medical technology developments of 2010 and it’s now coming to clinical use in the U.S
ALung Technologies’ Hemolung wins appreciations by using dialysis to perform respiratory gas exchange in a process similar to extracorporeal membrane oxygenation.
During therapy, the patient can stay awake. In February 2010, the first patient was successfully treated with the device. The system could revolutionize the practice of medicine in the ICU.
Advertisement