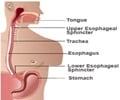
Ranitidine, the most popular acidity drug, widely used as Rantac, has found to contain a cancer-causing chemical.
According to the directive, ranitidine medicines contain a nitrosamine impurity called N-nitrosodimethylamine (NDMA) at low levels. NDMA has been classified by the International Agency for Research on Cancer (IARC) as probably carcinogenic to humans.
Ranitidine is used for multiple indications in the country and is available in different formulations, including tablets and injections. It is a prescription drug under Schedule H.
The state drug controllers have been asked to communicate the manufacturers of ranitidine to verify their products and take appropriate action for public safety.
The cancerous effect was noticed by the US FDA, which has issued an alert. Companies manufacturing the formulation in India are expected to stop production of ranitidine immediately. Following the Drugs Controller's directive, doctors would be advised by their medical associations to stop prescribing the medicine.
Source-IANS