Clinical Trial Studying Possible New Treatment Option for Patients with NAFLD
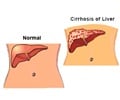
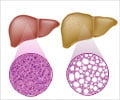
Go to source). According to the National Institutes of Health, approximately 24% of adults in the United States have nonalcoholic fatty liver disease (NAFLD), an umbrella term for a range of liver conditions affecting people who drink little to no alcohol that can lead to cirrhosis, liver cancer and liver failure.
‘Semaglutide, a medication used to treat diabetes and obesity, offers a promising treatment option for people with liver scarring. #FattyLiverDisease’





Currently, there are no medications approved by the U.S. Food and Drug Administration (FDA) to treat NAFLD. A recent $9.57 million grant awarded to researchers with the UC San Diego NAFLD Research Center (2✔ ✔Trusted Source
MASLD Research Center
Go to source) at University of California San Diego School of Medicine will support a clinical trial to study a new treatment option for patients with the disease.
Groundbreaking Insights on Liver Disease Detection and Management
“Liver disease is a silent killer, and most people do not know they have a liver problem until it is advanced to cirrhosis because there are no obvious symptoms,” said Rohit Loomba, MD, chief of the Division of Gastroenterology and Hepatology and director of the NAFLD Research Center at UC San Diego School of Medicine. “The results from our study could have a global impact on clinical care for patients with NAFLD and other chronic liver diseases.”Semaglutide belongs to a class of medications known as glucagon-like, peptide-1 receptor agonist (GLP-1 RA) that mimics the GLP-1 hormone released in the body’s gut in response to eating.
The randomized clinical trial will include 120 participants diagnosed with type 2 diabetes and obesity, who will either inject the drug or a placebo. Participants will administer the injection once a week and will follow a dose escalation schedule over a period of 16 weeks until they reach 2.4 mg weekly.
The participants will be screened with routine blood work and undergo a test for liver stiffness and liver fat using an ultrasound-based device in the patient’s primary care doctor’s office.
Advertisement
NAFLD and NASH — otherwise known as nonalcoholic steatohepatitis, is the most severe form of NAFLD and consists of excessive fat build up in the liver. Individuals who are overweight, have type 2 diabetes or have a family member with NAFLD are at a higher risk of developing the disease.
Advertisement
“Being awarded this grant represents an important step toward engaging populations in research, science and medicine to improve the health of individuals currently living with NAFLD,” said Loomba.
References:
- Clinical Trial Studying Possible New Treatment Option for Patients with NAFLD - (https://health.ucsd.edu/news/press-releases/2023-08-23-clinical-trial-studying-possible-new-treatment-option-for-patients-with-nafld/)
- MASLD Research Center - (https://medschool.ucsd.edu/som/medicine/divisions/gastro/research/NAFLD/Pages/default.aspx)