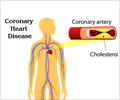
Treatment of non-ST-segment elevation myocardial infarction (NSTEMI) with anticoagulant fondaparinux had a lower risk of major bleeding events and death.

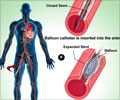
It was seen that the absolute rate of severe in-hospital bleeding events was lower in the fondaparinux group than the LMWH group (1.1 percent vs 1.8 percent), as was in-hospital mortality (2.7 percent vs 4.0 percent). The differences in major bleeding events and mortality between the two treatments were similar at 30 days and 180 days. The rate of recurrent heart attack was 9.0 percent in the fondaparinux group, and 9.5 percent in the LMWH group at 30 days and was 14.2 percent and 15.8 percent respectively at 180 days. The rate of stroke was low in both the groups. The rate of recurrent heart attack in the fondaparinux group was 9.0 percent vs 9.5 percent in the LMWH group at 30 days and was 14.2 percent vs 15.8 percent at 180 days. The rate of stroke was low in both groups. The results were consistent in patients with varying degrees of kidney function and in the subgroup of patients with NSTEMI who had undergone early percutaneous coronary intervention (a procedure used to open narrowed coronary arteries, such as stent placement).
The authors write, "A randomized clinical trial is often needed to provide definite evidence and an estimate of the treatment effect in a specific, selected, well-defined target patient population. However, the effect of implementing the same treatment in clinical practice might differ and should therefore be investigated in observational cohorts and, preferably, in continuous registries with complete coverage of nonselected patients with an indication for the studied treatment. Outside of a trial setting, the treatment is given to a much more heterogeneous patient population and the treating centers and physicians are less selected. Thus, the balance between benefit and risk can differ between a randomized clinical trial and experience in a nontrial, routine clinical care setting. Therefore, experiences from clinical practice provide important complementary information."
The study is published in 'JAMA'.
Source-Medindia