ATryn® or Antithrombin Recombinant safely prevents peri-operative and peri-partum acute deep vein thrombosis (DVT) in patients with hereditary antithrombin deficiency
ATryn® or Antithrombin Recombinant safely prevents peri-operative and peri-partum acute deep vein thrombosis (DVT) or other venous thromboembolic events in patients with hereditary antithrombin deficiency (HD AT), says a new study presented at the annual meeting of the International Society on Thrombosis and Haemostasis (ISTH) in Boston.
ATryn is not indicated for treatment of thromboembolic events in HD AT patients. Additionally, data validate dosing algorithms that, along with AT activity monitoring, allow physicians to normalize antithrombin levels during the high-risk situations of surgery and childbirth."ATryn provides physicians with a safe and effective new treatment option for restoring and maintaining antithrombin levels," said study co-investigator Michael Paidas, M.D., associate professor and director, Yale Women and Children's Center for Blood Disorders. "This is important because these patients have a very high risk of venous thromboembolism that can lead to serious and potentially life threatening complications."
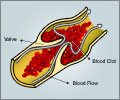
Antithrombin is a naturally occurring protein that helps regulate the blood clotting mechanism in the body. People with HD AT have reduced antithrombin activity, putting them at increased risk for venous thromboembolic events (VTE), including pulmonary embolism and DVT. These patients are at particular risk during surgery and childbirth procedures, when antithrombin levels are often low and need to be carefully managed.
"Data presented show that these validated, individualized dosing algorithms can be used in these different HD AT patient populations, which allows physicians to adjust antithrombin levels based on the fluctuating needs," said Dr. Paidas.
ATryn was created to provide a safe and reliable supply of recombinant antithrombin. Approved by the U.S. Food and Drug Administration (FDA) in February 2009 for the prevention of peri-operative and peri-partum thromboembolic events in patients with HD AT, it is the only recombinant form of antithrombin available in the world. Prior to the availability of ATryn, HD AT patients undergoing surgery or giving birth requiring an antithrombin therapy received treatment derived from human plasma. ATryn is not formulated with human plasma proteins.
Efficacy and Safety Results (Abstract PP-WE-405; Presented July 15, 2009)The first poster provided a pooled analysis of two Phase III studies (N=32) evaluating the efficacy and safety of ATryn in patients with HD AT undergoing surgery (11 patients) or childbirth procedures (21 patients). Patients were administered ATryn intravenously to maintain antithrombin activity within normal range (80% to 120%). The incidence of acute DVT or other venous thromboembolic events was assessed within seven days of treatment discontinuation. Safety was based on adverse events and laboratory evaluations.
Advertisement
The first poster also included a safety analysis which involved 47 patients from three studies (including a pharmacokinetic study). Adverse events were reported in 72 percent of patients and reflect those typically anticipated during the peri-partum and peri-operative period. Fifteen percent of patients reported an adverse event that was thought to be possibly related to ATryn, but none of these led to withdrawal from the study. Significant hemorrhage was reported in three patients: two of these episodes were associated with excessive heparin levels at the time of hemorrhage. The most commonly reported adverse events were anemia, vomiting, and headache (each 10.6%).
Advertisement
The analysis of AT levels from the first Phase III study (N=14: 9 pregnant and 5 surgical patients) that used the same dosing algorithm for pregnant and surgical patients found that pregnant HD AT patients have a higher clearance and volume of distribution than surgical patients, which led to the development of a specific dosing algorithm for pregnant patients.
The second study (N=18: 12 pregnant and 6 surgical) used a dosing formula based on patient type (pregnant or surgical) and, in most patients, AT level normalization markedly improved. Overall, a median of only one (range 0-6) infusion rate adjustment per patient was needed during treatment of a median of 3.2 days (range 0.9 – 14).
These studies support the use of separate dosing algorithms for pregnant and surgical patients.
Source-Eurekalert
RAS