Australian researchers have developed a monoclonal antibody to inhibit prostate cancer.
Scientists at Melbourne’s Burnet Institute, Melbourne, Australia have developed a monoclonal antibody to inhibit prostate cancer.
An article, which described the invention, has recently been published in the prestigious international journal the Journal of Clinical Investigation.
A monoclonal antibody is a laboratory-produced molecule that's carefully engineered to attach to specific defects in the cancer cells. Monoclonal antibodies mimic the antibodies the body naturally produces as part of the immune system's response to germs, vaccines and other invaders.
Over-expression of PIM-1 plays a critical role in the development, progression and metastasis of prostate cancer and other cancers such as leukaemia. The monoclonal antibody significantly developed by the Institute inhibited cancer cell growth when used in laboratory models of prostate cancer.
Professor Xing’s group demonstrated that their invention binds to PIM-1 present in cancer cells and creates a chain of events leading to the death of the cells. In particular, the therapeutic effect was improved by combination of the antibody with other drugs currently used to treat prostate cancer.
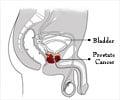
Prostate cancer is one of the most frequently diagnosed invasive cancers and the third leading cause of death in men worldwide. More than 3,000 men die each year from prostate cancer, equal to the number of women who die from breast cancer; and more than 18,000 new cases are diagnosed in Australia each year. A new strategy to treat prostate cancer is urgently needed as there is no efficient method to treat advanced prostate cancer.
Director of the Burnet Institute, Professor Brendan Crabb said that while the therapy was still in its early days this was the first time that researchers had found a treatment that targeted prostate cancer cells with a specific antibody to PIM-1 and which resulted in the death of the malignant cells and a reduction in tumour size.
Advertisement
While further laboratory research is still required to refine the treatment, it is expected that clinical trials of the new therapy will commence in the near future.
Advertisement
GPL/M

![Prostate Specific Antigen [PSA] Prostate Specific Antigen [PSA]](https://www.medindia.net/images/common/patientinfo/120_100/prostate-specific-antigen.jpg)