The New Zealand Ministry of Health has revealed that it will be withdrawing the type 2 diabetes drug, Rosiglitazone, from the market
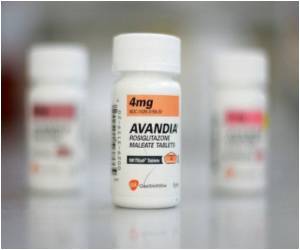
Now New Zealand is following EU’s footsteps with Medsafe Group Manager Dr Stewart Jessamine stating that the recently conducted safety review found that New Zealanders are at a greater risk of suffering from heart diseases on taking the drug.
“Patients who are taking Avandia are advised to make an appointment to see their doctor to discuss alternative treatments as soon as possible. Do not stop taking this medicine unless you have been advised by your doctor”, Dr Jessamine said adding that the drug will be withdrawn by April 29.
Source-Medindia