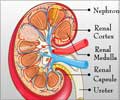
AWAK’s 3kg wearable PD device, now in trials, aims to bring end-stage kidney patients the freedom to live their lives on their own terms.
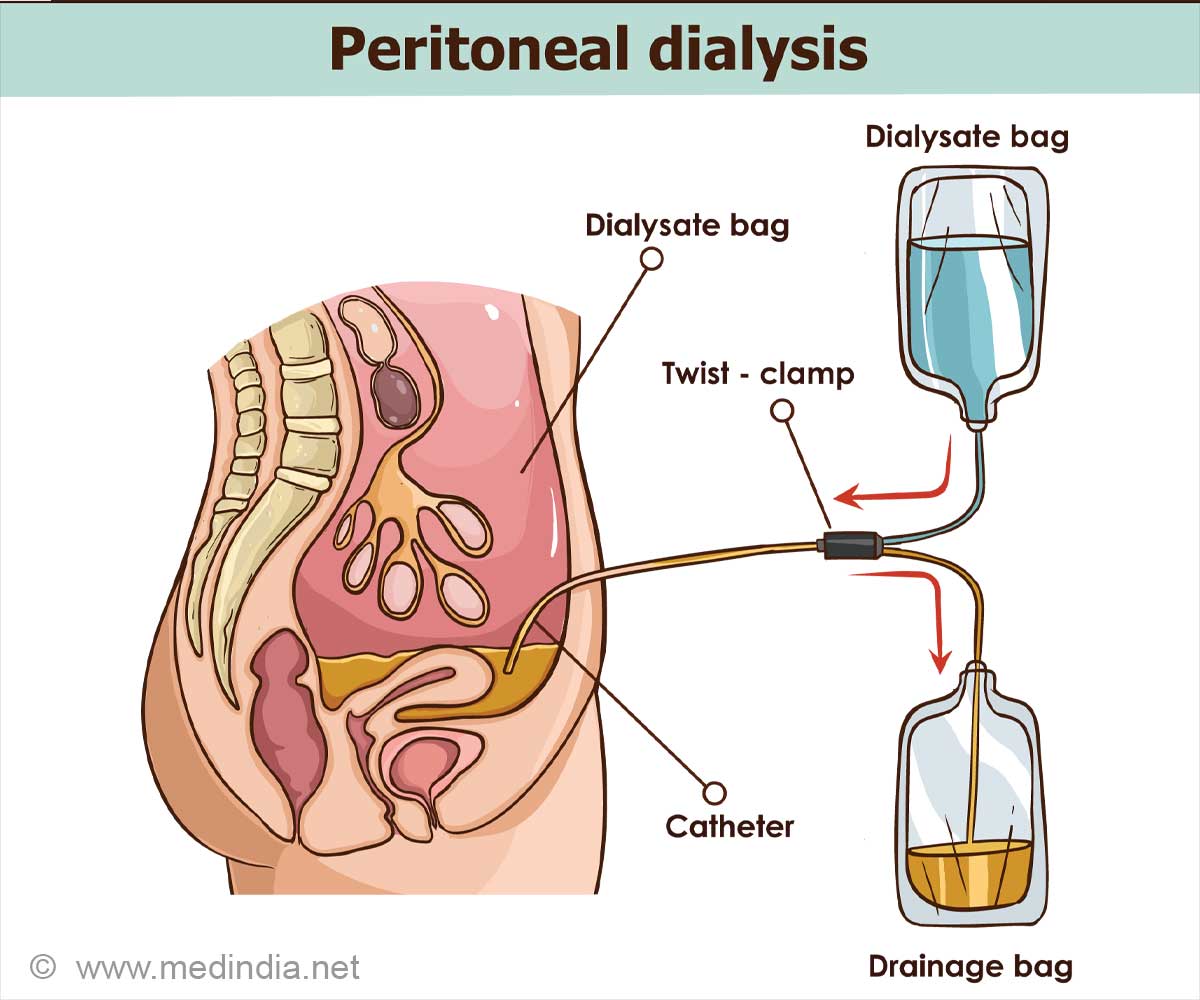
- AWAK Technologies’ wearable peritoneal dialysis (PD) device could allow kidney patients to perform dialysis on the go
- The device weighs 3 kg and eliminates the need for large dialysis machines, offering greater patient comfort and mobility
- AWAK PD has FDA breakthrough designation, and ongoing trials build on promising initial results with no significant adverse effects
AWAK and SGH begin wearable peritoneal dialysis device trial
Go to source). The single-arm, single-site, prospective experiment is a follow-up to the first-in-human study, which found no significant adverse effects.
New Portable Dialysis Device for End-Stage Kidney Disease
AWAK PD trials chief investigator associate professor Marjorie Foo stated, "We have worked with AWAK Technologies on this study since its early stages. Despite COVID-19 slowing us down, we have continued to improve on the device and are now ready to embark on a pre-pivotal trial, one step closer to what our patients are hoping and looking forward to a life on dialysis that will minimally affect their lifestyle and yet provide good quality dialysis." AWAK PD is a wearable and ultra-portable peritoneal dialysis (PD) technology that allows patients with end-stage kidney disease to get dialysis on the road.Portable peritoneal dialysis (PD) technology uses patented sorbent technology to make dialysis on the go possible- recycling dialysate in real time, so you can enjoy more of life beyond the hospital! #healthcareinnovation #dialysistech #medindia’
Portable Dialysis Solution Provides Greater Freedom and Comfort to Kidney Patients
The tiny device, weighing approximately 3 kg, incorporates the company's patented sorbent technology to modify the current method of administration for peritoneal dialysis. The technology is expected to assist overcome the difficulty of connecting to huge dialysis machines and enduring long periods of stationary therapy in clinics and hospitals.The business obtained breakthrough device designation from the US Food and Drug Administration for the gadget. AWAK Technologies CEO Suresha Venkataraya stated, "Entering the pre-pivotal trial with the enhanced version of our device represents a significant step in our mission to revolutionise the dialysis industry. The invaluable insights and feedback from the initial first-in-human trial have been vital in refining and enhancing our product."
Reference:
- AWAK and SGH begin wearable peritoneal dialysis device trial - (https://www.medicaldevice-network.com/news/awak-sgh-begin-wearable-peritoneal-dialysis-device-trial/)
Source-Medindia