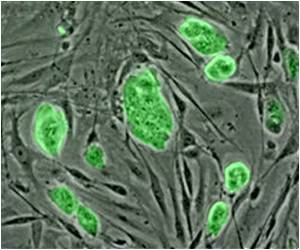
A study has found that transplanting self-donated Schwann cells (SCs, the principal ensheathing cells of the nervous system) that are elongated so as to bridge scar tissue in the injured spinal cord, aids hind limb functional recovery in rats modeled with spinal cord injury.

"Although numerous cell transplantation strategies have been developed to nullify the lesion environment, scar tissue - in basil lamina sheets - wall off the lesion to prevent further injury and, also, at the interface, scar tissue impedes axon regeneration into and out of the grafts, limiting functional recovery."The researchers determined that the properties of a spinal cord/Schwann cell bridge interface enable regenerated and elongated brainstem axons to cross the bridge and potentially lead to an improvement in hind limb movement of rats with spinal cord injury. Electron microscopy revealed that axons, SCs, and astrocytes were enclosed together within tunnels bounded by continuous basal lamina.
The expression of neuroglycan (NG2; a proteoglycan found on the membrane of cells) was associated with these tunnels. They subsequently determined that a "trio" of astrocyte processes, SCs and regenerating axons were "bundled" together within the tunnels of basal lamina."Elongation of astrocyte processes across transplant interfaces likely establishes three-dimensional structures that determine how regenerating axons become exposed to myriad growth-promoting and inhibitory cues," wrote the researchers. The researchers also noted that it was important to understand conditions that favor astrocytes to be permissive for axonal growth into lesion transplants.
"We demonstrated that the elongation of astrocyte processes into SC transplants, and the formation of NG2+ tunnels, enables brainstem axon regeneration and improvement in function," they concluded. "This study supports the clinical use of SCs for SCI repair and defines important characteristics of permissive spinal cord/graft interfaces."
"Developing the means to bridge the glial scar following chronic spinal cord injury is one of the major stumbling blocks of therapy" said Dr. John Sladek, Cell Transplantation section editor and professor of neurology and pediatrics at the University of Colorado School of Medicine. "This study provides important new insight into how this may be achieved".
Source-Eurekalert